Understanding the difference between Orbit and Orbital is very useful for those interested in physics. The basic definitions of orbit and orbital explain the difference between the two; An orbit is defined as a specific path on which the electrons revolve, while the orbital is an indistinct area, and the chance of finding an electron here is maximum. Orbit is considered as a two-dimensional or a planar area. But, the orbital represents a three-dimensional area in which the probability of the presence of an electron is maximum.
Basics of Orbit and Orbital
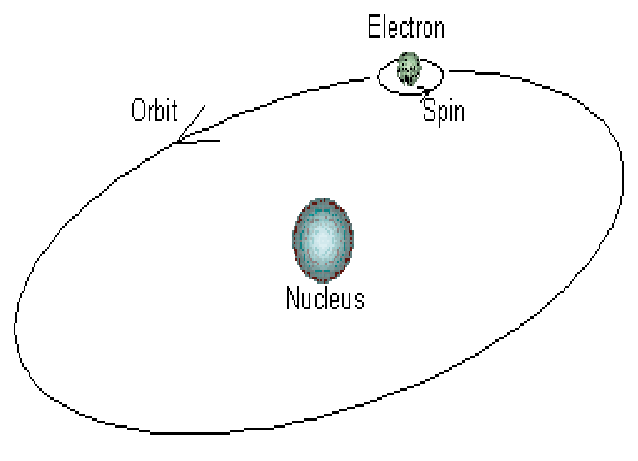
Electrons in an atom can be compared to planets in our solar system, with the nucleus representing the sun. When electrons spin around their orbit, they follow the same principles that planets do when they orbit the sun.
Since electrons revolve around the nucleus, we can pinpoint regions of space surrounding the nucleus where there is a high possibility of finding an electron. The location of an electron cannot be precisely defined, according to Heisenberg’s uncertainty principle. The idea of the orbital is developed to describe the location of an electron inside an atom. As a result, instead of definite orbits, Bohr proposes the idea of orbitals. An orbital is the area of space surrounding the nucleus where the likelihood of finding an electron is greatest. The depiction of orbitals in diagrams is challenging.
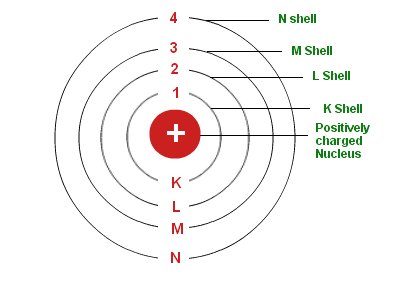
Electrons circulate around the nucleus only in specific circular routes known as orbits. These orbits are referred to as energy levels, energy shells, or quantum levels since they are associated with certain energies. These levels are numbered as 1, 2, 3, 4, etc, or labeled K, L, M, N, etc.
The orbital does not specify an electron’s speed, direction, or position. However, it provides a precise estimate of where an electron may be.
Orbit vs. Orbital In Terms of Definitions
The main difference between orbit and orbital refers to their definitions. In this section, we will briefly describe the primary concepts of these two scientific terms.
Orbit
Physicists define an orbit as the gravitationally curved path of an object, such as a planet’s trajectory around a star or a natural satellite’s path around a planet. As a rule, orbits are repeating trajectories, but they can also be non-repeating trajectories. Kepler’s laws of planetary motion describe planets and satellites’ motion following elliptic orbits, with their center of mass orbiting the focal point of the ellipse.
By the definition of the term orbit, it is a circular track on which electrons revolve due to the force exerted by the positively charged nucleus on the electrons. That’s what Bohr’s atomic theory says.
According to Bohr’s model, the first shell of an atom contains only two electrons. Bohr’s model was, however, later rejected. It is the orbital model that is widely accepted at the present time. Knowing how the solar system works is essential in order to understand an orbit. In this sense, the planets of the solar system are electrons that orbit the sun, which is the nucleus in this case. The electrons, like the planets, do follow Newton’s laws of motion as they revolve around their nucleus. The path of an orbit, in contrast to an orbital, is only two-dimensional.
Heisenberg’s uncertainty principle says that the position of an electron cannot be determined accurately. This is where the orbital concept comes into play.
Orbital
Atomic orbitals are mathematical functions that explain the location and wavelike behavior of electrons within atoms in atomic theory and quantum mechanics. It can be used to calculate how likely it is that an electron will be found in a certain region around an atom’s nucleus. Atomic orbitals may also refer to the physical space or region where, according to the particular mathematical form, the electron is predicted to reside.
Each orbital of an atom is distinguished by a unique set of quantum numbers n, l, and ml, which reflect the electron energy, angular momentum, as well as velocity vector component of the electron (the magnetic quantum number). A maximum of two electrons can fill each of these orbitals, each with its own projection of spin, i.e., ms.
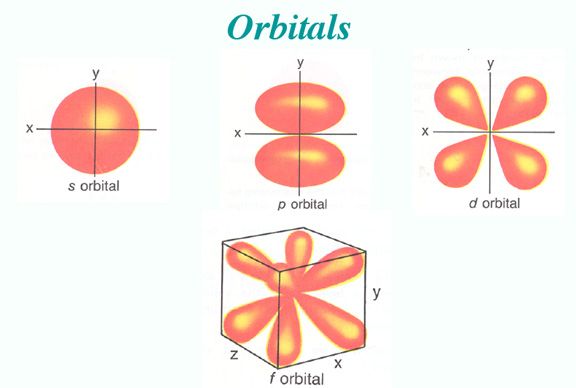
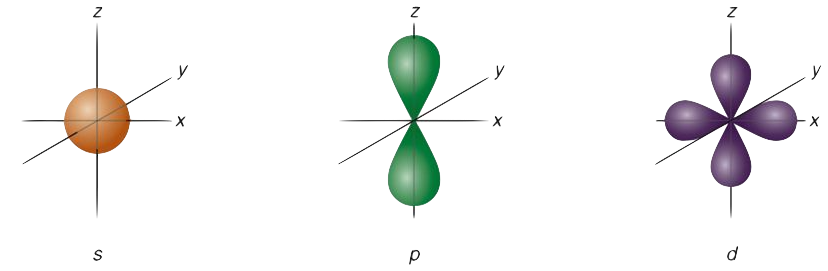
The s orbital, p orbital, d orbital, and f orbital all denote orbitals in which the angular momentum quantum number l is 0, 1, 2, and 3, respectively. Together with n, these names describe the electron configurations of atoms. Initially, they were described by general spectroscopists as sharp, principal, diffuse, and fundamental lines based on the spectroscopic properties of alkali metals. The orbitals for l > 3 continue alphabetically without the letter “j” (g, h, i, k, …) since some languages do not separate “i” from “j.”
An interpretation of submicroscopic electron behavior in the matter is based on atomic orbitals (alternatively known as electron clouds or wave mechanics). Atomic orbitals constitute the fundamental building blocks of this visualization. According to this model, the electron cloud of a multi-electron atom is built up from simpler hydrogen-like atomic orbitals (in approximation).
A summary of these sections regarding the orbital can be summarized as follows: In the theory of Heisenberg, it is impossible to determine the exact position of an electron. This is how we begin to understand the orbital. An orbital is an uncertain area where the probability of finding an electron is greatest. Basically, the orbital space represents the three-dimensional space around the nucleus. The probability of finding an electron in the three-dimensional region of an atom is extremely high, about 95 percent. The molecular shape can be easily determined when we use orbitals since they are directional.
Orbit vs. Orbital In Terms of Accuracy of Electron Positioning
Atomic orbitals do not seek to describe electron positions precisely within an atom, while orbit aims to describe electron positions with precision.
Orbit vs. Orbital In Terms of Uncertainty Principle
As orbits provide information about an electron’s exact location, they do not follow Heisenberg’s Uncertainty Principle. In contrast, orbitals do not accurately represent electron positions, so they follow Heisenberg’s Uncertainty Principle.
Orbit vs. Orbital In Terms of Shape
Every orbit has a circular shape. The orbital, on the other hand, can take many forms, including spherical and bell-shaped forms.
Orbit
Electrons, unlike planets orbiting the Sun, must be within certain specific distances from their nuclei to exist; these are called allowed orbits. In 1913, Danish physicist Niels Bohr first explained the quanta-basis property of electrons, which states that, like everything in the quantum world, the angular momentum of electrons is a discrete bundle called quanta.
An electron can only be found in allowed orbits in the Bohr atom, and these allowed orbits have different energies. It is analogous to a set of stairs on which the gravitational potential energy varies from step to step with a ball on any step but not between.
Orbits are predictable paths taken by electrons. As a modification of Rutherford’s model, Bohr explained that electrons move about within fixed orbital shells. Thus, Rutherford had laid out a basic concept of atom nucleus, whereas Bohr had taken it a step further. The electrons and their different energies are explained by him.
Bohr’s atomic model assimilates the concept of a small, positively charged nucleus surrounded by orbiting, negatively charged electrons. Based on his findings, electrons will have more energy if they are located far from the nucleus, while electrons will have less energy if they are located near the nucleus.
There will be a fixed amount of energy in each circular orbit, which is referred to as an orbital shell. As long as the electrons remain in fixed orbital shells surrounding the nucleus, they won’t radiate energy. Quantum numbers differ in the range from the lowest energy level (nucleus side n=1) to the highest energy level.
Two different ways of representing energy levels or orbits are 1, 2, 3 or K, L, M, N,… shells. It is at the ground state that the electron has its lowest energy level.
Electrons change energy levels as they move from one level to another. By acquiring energy, electrons move from a lower to a higher energy level in an atom. In contrast, if an electron loses energy, it drops from a higher to a lower energy level.
1st Orbit
Known as the K shell, it can hold up to two electrons.
2nd Orbit
The L shell can accommodate up to eight electrons.
3rd Orbit
This shell has up to 18 electrons and is represented as an M shell.
4th Orbit
It is shown as N Shell, and it can hold a maximum of 32 electrons.
Orbital
An atom has a large number of orbitals. Atomic orbitals can take many different shapes. Having a small orbital means that the electron will be more likely to be found near the nucleus. The shapes and orientation of atomic orbitals indicate that the electron is unlikely to be found in some directions more than in others.
In other words, orbitals are the areas of space in which electrons usually exist. Numerals and letters are used to identify the orbitals. Numbers in this context refer to the levels of energy held by electrons in an orbital.
Number 1 is the level of energy closest to the nucleus, and number 2 is the next level of energy further away from the nucleus. Originally, the letters s, p, d, and f stood for sharp, principal, diffuse, and fundamental. Using this method, the spectral lines of different atoms can be determined.
S Orbital
Using the boundary surface diagram for the s orbital, we can see a sphere with the nucleus as its center. In two dimensions, it looks like a circle. As a result of their spherical symmetry, a given distance will result in the same probability of finding electrons from all directions. The size of the s orbital will increase as the quantum number n increases.
P Orbital
Typically, a p orbital is composed of two parts known as lobes, which lie on either side of the nucleus plane. In all three p orbitals, the lobes are oriented differently. However, their size, shape, and energy are very similar. These lobes lie along each of the x, y, and z-axes and are given descriptions 2px, 2py, and 2pz.
Therefore, there are three p orbitals whose axes are perpendicular to each other. Similar to the s – orbitals, when the p orbital quantum number increases (4p > 3p > 2p ), the size and energy of the p orbitals also increase.
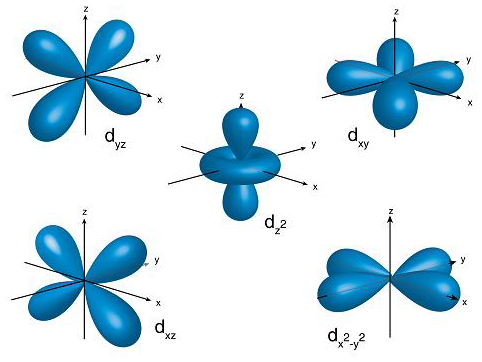
D Orbital
For d-orbitals, the magnetic orbital quantum number is defined as -2, -1, 0, 1, 2. We can say that there are 5 d – orbitals which can be designated as 5dxy, 5dxz, 5dyz, 5dx2-y2, and 5dz2. There are five d orbitals; four of them have a similar shape to each other, whereas one dz2 orbital differs from the other. However, all five d orbitals have the same energy.
Orbit vs. Orbital In Terms of Designation
A number of letters are used to name orbits, such as K, L, M, and N. The term orbital, however, is identified by using the letters s, p, d, and f.
Orbit vs. Orbital In Terms of Direction
The orbits show no characteristics to indicate a direction, but the orbital does, except for the s orbital, show directional characteristics.
Orbit vs. Orbital In Terms of Capacity for the Placement of Electrons
An orbit can accommodate electrons equal to the following:
Number\ of\ electrons\ in\ an\ orbit=2n^2
Where n is the orbit number or the shell number. For example, K shell would represent the first orbit and the L shell would represent the second.
An orbital can accommodate no more than two electrons in its sub-levels. There is only one sub-level in the s orbital, so it can contain only two electrons. However, the p orbital has three sub-levels, so it can hold up to six electrons.



