A spectrophotometer can be located in many studies, biology, chemistry, and industrial laboratories. The spectrophotometer is utilized for research and data evaluation in different scientific fields. It offers a high degree of precision, sensitivity, and accuracy. In addition, it is inexpensive and applicable to the measurement of a variety of substances. What a spectrophotometer does is transmit and receive light. The spectrophotometer is utilized to evaluate samples of test material by passing light employing the sample and studying the intensity of the wavelengths.
Different samples modify the light in numerous distinct ways and this allows researchers to obtain much more facts about the check content, by viewing the change in light conduct as it passes by way of the sample. If you are eager to know about how does a spectrophotometer works, read this new blog in Linquip to find out more about it.
Components of Spectrophotometer
There are different types of spectrophotometer available but all of them has similarity in structure and parts. Before getting to know how a spectrophotometer works, Let us overview the components of this device.
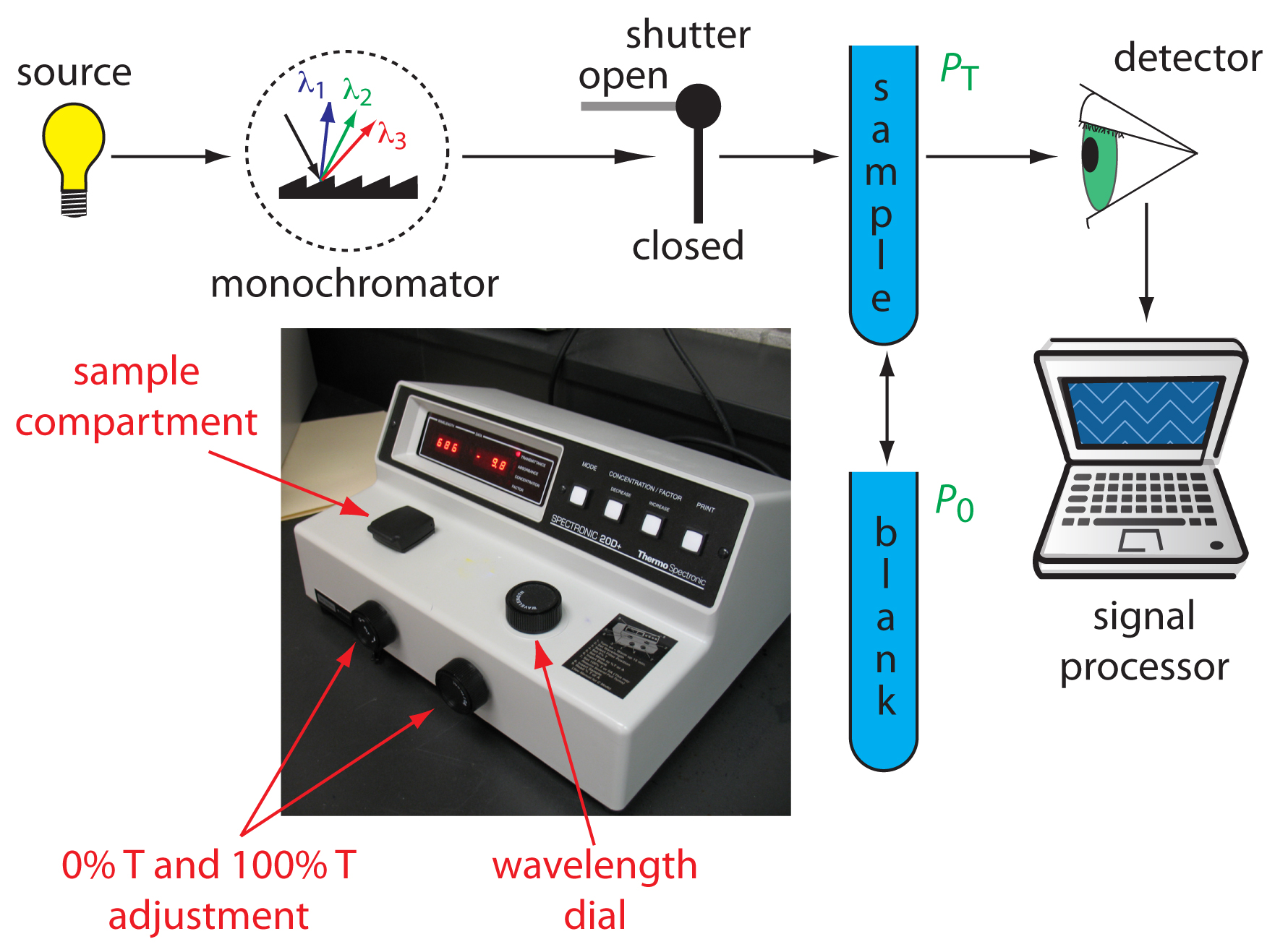
- Lamp: light source(s)
- Monochromator: a means of isolating a particular wavelength band of the light source, provide Monochromatic light which is light in which all photons have the same wavelength
- Cuvette or cell: a sample holder
- Detector: a device to measure light intensity
- Display: displays both transmittance and absorbance of a sample
Working Principles of Spectrophotometer
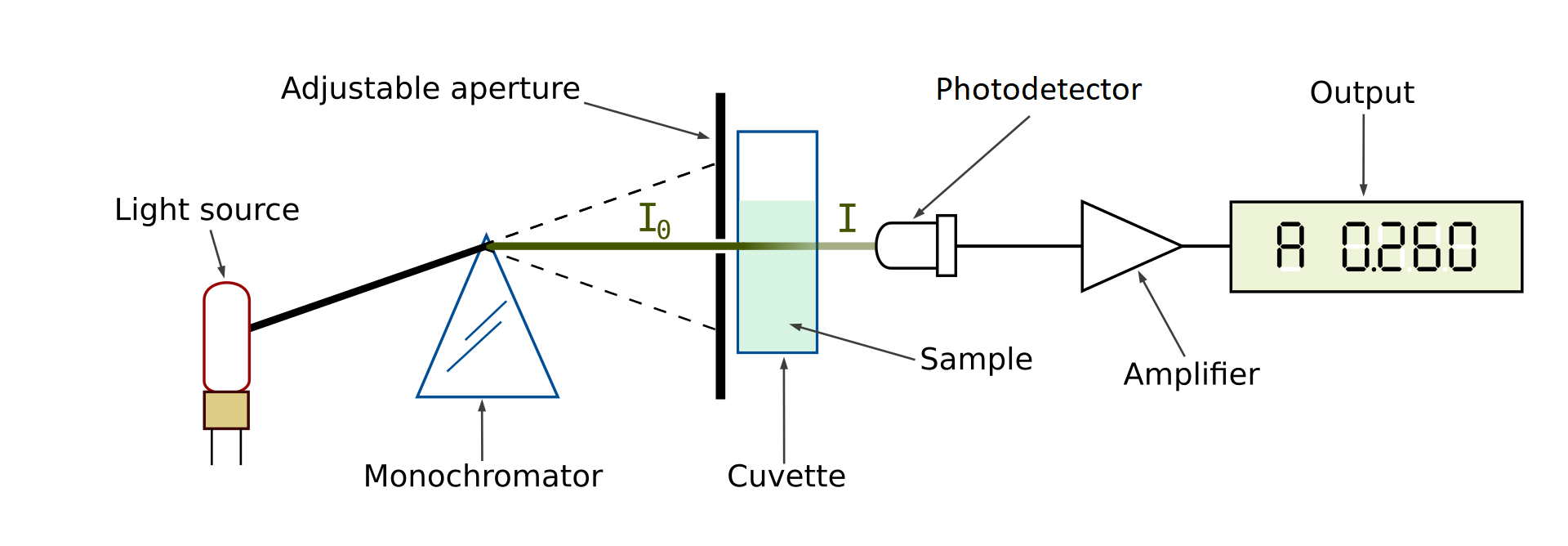
There is an interaction between electromagnetic radiation (light) and matter. A spectrophotometer consists of two instruments, namely a spectrometer for producing light of any selected color (wavelength), and a photometer for measuring the intensity of light. The amount of light passing through the tube is measured by the photometer. The photometer delivers a voltage signal to a display device, The signal changes as the amount of light absorbed by the liquid changes.
Performance Figures of Spectrophotometers
The most important performance characteristics of such instruments are:
- The quantities which can be measured (e.g. absorbance only, or absorbance and reflectance)
- The accessible spectral range, ideally UV – VIS – IR
- The spectral resolution (fixed or variable, often limited by a monochromator)
- The absorbance range (limited by the light source and the detector sensitivity)
- The measurement time for a given spectral range and resolution
How Does a Spectrophotometer Work?
Here’s how a spectrophotometer works. A sample solution is placed inside the spectrophotometer. A lamp provides the source of light. The beam of light strikes the diffraction grating, which works like a prism and separates the light into its component wavelengths. An adjustable slit allows only one specific wavelength of light through to the sample solution. Then the light interacts with the sample. The wavelength of light hits the sample, which is held in the cuvette. We need to be careful when handling cuvettes; even a slight fingerprint can interfere with the results. From this point, the detector measures the transmittance and absorbance of the sample.
Transmittance refers to the amount of light that passes completely through the sample and strikes the detector. Absorbance is a measurement of light that is absorbed by the sample. The detector senses the light being transmitted through the sample and converts this information into a digital display on the output screen.
Steps In Working With a Spectrophotometer
- When warming up the spectrophotometer, there should be no cuvettes in the machine.
- Preparation of samples
- A series of standard solutions of known concentration
- Set the spectrophotometer to the wavelength of maximum light absorption. Measure light absorbance of standards.
- Set the percentage transmittance of light as 0%
- In the sample space, lodge a cuvette, filled with solvent and close the sample space.
- Set the transmittance at 100%
- For comparison, fill the cuvette with the sample and place it in the sample space and close the sample space.
- Note down the reading on the Photometer for calculations.
- Plot standard curve: Absorbance vs. Concentration.
- Calculating the concentration of the sample using Beer-Lambert Equation: A = ECL
So, there you have every single fact about the spectrophotometer function. If you enjoyed this article in Linquip, let us know by leaving a reply in the comment section. Is there any question we can help you with? Feel free to sign up on our website to get the most professional advice from our experts.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More In Linquip
- A Cheat Sheet For Types of Spectrophotometers
- Want To Know Everything About Spectrometers vs Spectrophotometers? Now You Can!
- Beginners Guide: What Is a Spectrophotometer
- The Difference Between Colorimeter and Spectrophotometer
- A Quick Look at Types of Spectrophotometers
- Spectrophotometer VS Colorimeter: Which Do You Need?