“How does nuclear fusion work” is one of the fundamental questions of physics to discover the nature of the universe. Scientists broke the atom in the early 20th century and thought they were revealing the mystery of world creation from basic pieces of matter. What they did not recognize in those days was that they had discovered an entirely new idea of energy production that would be used in atomic bombs and nuclear power plants.
Ernest Rutherford is the scientist who led the first atom-smashing experiments.
Today, nuclear power is widely used in many countries, even though it is hugely expensive and politically questionable. It has caused some catastrophic disasters, and sea pollution, and has left terrible masses of highly fatal radioactive waste.
Nuclear power plants take energy by splitting large atoms through a process called nuclear fission. Future plans may operate using a completely distinct form of nuclear power; this way is called nuclear fusion, where small atoms are forced to build large ones. This is much cleaner and more reliable and could likely solve our energy demands forever. To answer the question of “how does nuclear fusion work,” it is better to first look at the physics of this phenomenon.
Physics of Nuclear Fusion
Nuclear fusion is the most powerful physical process in the universe. In this process, two very excited, very energetic, and very hot atoms collide with each other and become one atom, releasing a few subatomic particles and the remaining energy in the process.
After the Big Bang, the whole world was an extremely hot and energetic soup of very small subatomic particles, except that they could not yet be called subatomic particles because atoms did not exist at this stage. Eventually, these tiny particles began to absorb each other and bond, converting quarks into electrons, neutrons, and protons—the basic building blocks of matter. The hot and dense soup began to cool and thicken as it expanded, building small lumps of hydrogen gas.
For a time, the universe was only the simplest element, i.e., hydrogen. However, gravity slowly began to gather some of these gas clouds together. As the hydrogen atoms zipped in and gained more energy in their denser and hotter environment, they fused to form the second lightest element, i.e., helium.
Many gas clouds formed various stars, including the Sun, as enormous spheres of hydrogen and helium plasma. In the dense centers of these stars, hydrogen and helium proceeded to fuse until they made heavier elements. When the early stars of the universe died and erupted into nova and supernova, they released clouds of heavier elements into space that eventually became the planets, asteroids, comets, and other interstellar objects.
It takes a lot of energy to cause nuclear fusion. Atomic nuclei containing positively charged protons and neutral neutrons do not tend to come close to each other under typical conditions. The Coulomb force, which explains how similar charges repel each other and opposite charges are absorbed (such as the south and north poles of a magnet), prevents the two atomic nuclei from colliding with each other.
Suppose you put two atoms on a direct collision path so that their nuclei smash and stick together. In that case, you must accelerate them to very high speeds so that when they collide, the nuclear force that coerces protons to stick to the neutrons exerts the repulsive Coulomb force.
Nuclear binding energy is the minimum energy required to destroy an atomic nucleus. The denser the element, the more energy is needed to disintegrate its nucleus. With nuclear fission or fusion, the energy of nuclear binding is released. In this way, nuclear fission and fusion can be utilized to generate electricity.
For more massive elements, fusion does not release energy. However, in the case of lighter elements, like hydrogen and helium, with the combination of two atoms, the resulting third atom is filled with extra energy and an extra neutron or two in its nucleus, which makes it unstable. No atom ever wants to be unstable, so it tries to go to the nearest point of stability by releasing extra energy. It relieves itself by expelling out the excess neutron(s) with its remaining energy.
Nuclear Fusion in Nature
It is not directly apparent, but almost everything you do now (like breathing in and out) and everything you can see nearby is powered by nuclear fusion because that is what moves the Sun, and the Sun causes the Earth to move.
If you could get close to the Sun to see inside its core and see what happens inside it, you would see hydrogen atoms come together (so-called fusing) to build atoms of helium, releasing a lot of energy during the process. In addition to producing incredible heat inside the Sun itself (the temperature of the core is at least 10 million degrees), the Sun’s nuclear fusion also produces the solar energy that flows through 150 million kilometers (93 million miles) of space and also sustains all the life existing on the Earth.
Imagine that we could build a device that copies what is happening inside the Sun on Earth in a much smaller and more manageable way. Theoretically, we can only feed in hydrogen (a simple, relatively safe gas we can produce from water) on one end, smash its atoms together, and receive helium (a safe and clean gas) out on the other end.
In this process, we will generate a large amount of thermal energy, which can be used to run steam turbines and generators to take electricity, just as in any conventional power plant. There will be no pollution, no carbon dioxide (a greenhouse gas that causes adverse effects like global warming and climate change), and no lethal nuclear waste. We will have simple, clean, and safe nuclear power!
Nuclear Fusion on Earth
There is a huge difference between the core of the Sun and inside a power plant on Earth. So, it is a challenge to find a practical way to make nuclear fusion reactions. It seems that instead of using ordinary hydrogen atoms, the best way is to work with other slightly heavier isotopes of hydrogen. A typical hydrogen atom has only one proton in the nucleus and one electron in its surrounding, but these heavier hydrogen isotopes are made differently (and therefore much less stable).
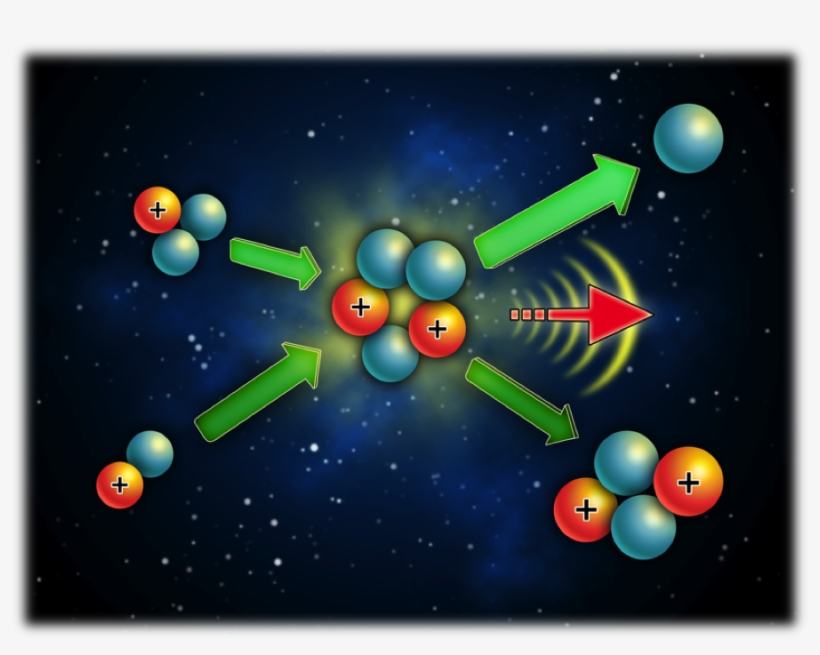
Deuterium is one of them that has one proton and one neutron in the nucleus and one electron in outer space. The other one is tritium, including one proton and two neutrons in the nucleus, and one electron outside.
By smashing together an atom of deuterium and an atom of tritium, the pieces we have to play with include two protons, three neutrons, and two electrons. Therefore, we have a very stable atom of helium, including two protons, two neutrons, two electrons, and one neutron left over. By turning two unstable atoms (each of deuterium and tritium) into a stable helium atom, we release a large amount of energy.
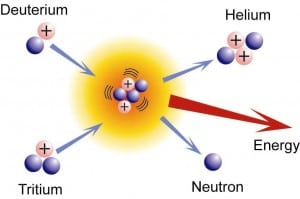
Chemical reactions can release energy if the reaction products are more stable than the reactants. However, extremely more energy from a nuclear reaction can be obtained than from a chemical reaction.
During a nuclear reaction, the atoms you begin with are converted into entirely different atoms. However, in a chemical reaction, the atoms keep their characteristics but combine or rearrange into different molecules. So, the amount of energy needed to retain the nucleus of one atom together is much greater than the amount of energy in binding two different atoms.
The Source of Nuclear Energy (Heading 3)
The atom nucleus is held together because it is more stable than its separate parts. When those parts are put together, energy is released. The energy is related to the fact that the nucleus mass is less than the mass of the items it is made from (protons and neutrons or “nucleons”). The difference between the two masses is equal to the binding energy. This directly follows two things. The first comes from Einstein’s idea that mass and energy are equivalent, represented by the following equation:
E=mc^{2}
The Second is the law of conservation of energy that states mass or energy cannot be destroyed; only they can be changed into other forms.
Different nuclei have various numbers of protons and neutrons that come together more or less efficiently, which means they have different binding energy levels. This is why switching from one atom to another one releases energy from the nucleus or nuclear energy. In other words, in nuclear fusion, we connect small unstable atoms to larger and more stable atoms to release binding energy.
Particles in the atom nucleus are influenced by two main forces: the powerful nuclear force acting over short distances, which attracts them together, and the repulsive electromagnetic force over long distances, which propels them apart.
Small atoms can become more stable by clumping together into larger units to get the most out of stronger nuclear force. Large atoms can split into smaller ones to stabilize themselves to reduce the repulsive electromagnetic force. The former causes fusion and the latter causes fission.
The amount of Released Energy in a Fusion Reaction
Comparing the mass of a deuterium atom and a tritium atom (as the reactants) with the mass of a helium atom and a neutron (as the products) results in the point that some mass is lost in the fusion reaction. The lost mass is converted into energy based on Einstein’s equation mentioned before. It shows that a small amount of mass can generate a large amount of energy because the value of c, the speed of light, is large, and c squared is even bigger.
The amount of released energy in a single reaction is 17.59 Megavolts (17.59 million electron volts), a confusing measurement that only physicists use and understand. To be more meaningful, you need about 3.5 million of those reactions per second to turn on a low-energy 10-watt light bulb. Remember that 1g of pure hydrogen contains 600,000 million million million atoms, and you can see that potentially a lot of fusion power with very little fuel.
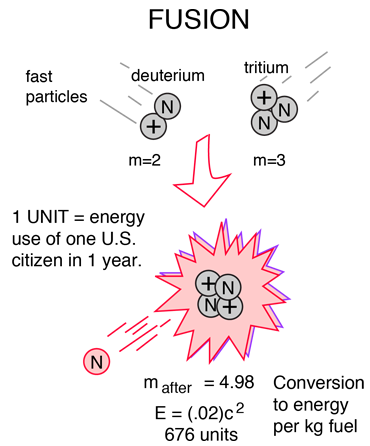
If we consider deuterium and tritium, the figures are slightly different, but the basic point—a small amount of matter containing a large number of atoms is still preserved.
How Does Nuclear Fusion Work in Practical Plants?
As described, the basis of nuclear fusion on Earth is transforming deuterium and tritium to helium, with a release of considerable energy as two unstable atoms rearrange themselves to produce one stable atom. Although it may seem simple enough, no one has yet been able to make fusion work on a scale large enough to generate commercial power. This is because there are huge practical problems.
To fuse two atoms, you have to move their nuclei closer and closer. The problem is that the nucleus of each atom has a relatively large positive charge, so two nuclei repel each other as magnet poles repel.
The closer the nuclei get, the more energy is needed to get them closer, and the amount of energy continues to increase. The repulsive force existing between the two nuclei quadruples each time you halve the distance because of Coulomb’s Law. In practice, this means that you have to use much energy to fuse two atoms to release even more energy finally.
So far, scientists have not reached the break-even point to release something as energy is consumed. This is why nuclear fusion is currently only being studied in scientific laboratories and why at least now, this is not a practical source of power provision.
Confinement
To make atoms fuse, scientists heat them and produce plasmas, i.e. ultra-hot soups of gases in which the atoms become so hot that they are separated and exploded in their constituent nuclei electrons. Then they keep them firmly in a confined space at a temperature at least as hot as in the center of the Sun.
So, the problem of energy production by fusion is different: how can we limit a super-hot plasma to staying hot and energetic enough so that the atoms inside it can overcome their natural repulsion and fuse? In stars like the Sun, the force of gravity gains this. On Earth, we have to artificially reach the same goal. There are two basic means to do this.
Magnetic Confinement
In a method called magnetic confinement, deuterium and tritium are heated to a temperature of about 100 million degrees. An extremely strong magnetic field is then used to trap them in the form of a donut known as a torus.
The device for doing this is known as a Tokamak; a word made just for this piece of machine in Russia. The largest in the world currently operates in the JET laboratory (Joint European Torus) in England.

Inertial Confinement
The other fusion method is known as inertial confinement and includes firing a powerful laser at a fuel pellet. Then the atoms inside it are immediately heated up and fused. The fuel burns before it is destroyed, so it effectively limits its mass and its inertia. An example of this system is operating at NIF (National Ignition Facility) in California. In this mechanism, 192 laser beams (which make up the world’s largest laser) are fired simultaneously into a small fuel pellet to produce the right conditions for nuclear fusion.
The figure below shows the Schematic of the inertial confinement fusion stages using lasers which are explained in what follows.
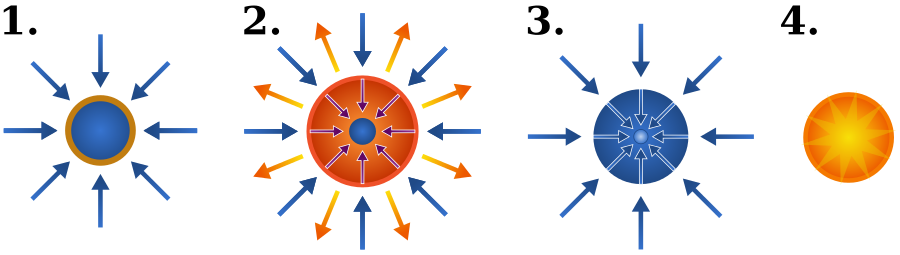
- Laser beams quickly heat the surface of the fusion target to form a plasma envelope.
- The compression of the fuel occurs by the rocket-like blowoff of the surface.
- During the final section of the capsule implosion, the fuel density reaches 20 times that of lead and ignites at 100 million degrees Celsius.
- Thermonuclear burn diffuses rapidly through the compressed fuel, generating many times the initial energy.
Although impressive, JET and NIF are still just laboratory tests. Hopes of attaining anything like commercially viable fusion are now tied to another international project known as ITER, which is now under development in the south of France. It is expected by the end of the project in around 2025, it should be able to produce about ten times more energy than it utilizes for up to about 8–10 minutes.
Nuclear fusion holds great promise for the future: vast reserves of clean energy from readily available fuels that are unlimited. It does not cause any nuclear waste or nuclear pollution (except for reactor equipment, which continues contaminated for about a century at the end of its life) and does not emit greenhouse gases.
There is no risk of nuclear accidents like those of fission plants (in terrible accidents such as at Chornobyl, Ukraine in 1986, and Fukushima in 2011). However, unfortunately, scientists have estimated that we are decades away from seeing commercial fusion plants.
What is The Difficulty of Nuclear Fusion?
A fundamental challenge is establishing a structure strong enough to hold the plasma under the large values of required pressure. The UK Atomic Energy Authority chief executive believes that Exhaust systems must withstand heat and power similar to a re-entering spaceship orbit. Robotic maintenance systems as well as systems for breeding, recovering, and fuel storage will be required.
History of nuclear fusion development
From the 1890s until the 1920s, Sir Ernest Rutherford, a New Zealand scientist, and colleagues demonstrated nuclear fission and nuclear fusion in a long series of innovative physics experiments that gradually exposed the structure of the atomic nucleus. In 1920 Sir Arthur Eddington, a British physicist suggested that stars like the Sun get their energy from fusion. Atomic scientist Hans Bethe used these ideas in 1939.
Sir Mark Oliphant, an Australian-born scientist, demonstrated nuclear fusion in a laboratory for the first time in 1934. Soviet nuclear physicist Andrei Sakharov 1950 designed a particular reactor to create fusion, named Tokamak. Thus the USSR’s fusion program began. An American, Lyman Spitzer, invented an alternative plasma-confinement reactor, a stellarator, in 1951.
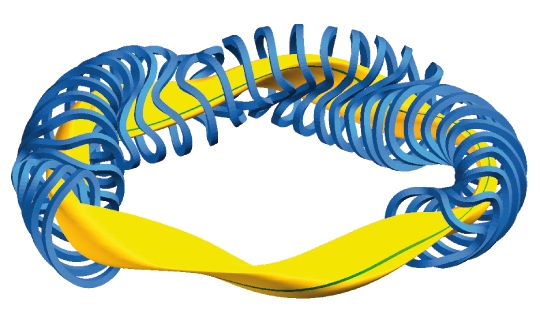
The first Tokamak is created in 1958. In 1997, the National Ignition Facility (NIF) fusion experimental project started at Lawrence Livermore National Laboratory in California. In 2013 building the International Thermonuclear Experimental Reactor (ITER) in France started and is currently expected to be finished in 2025. In 2018 MIT researchers estimated that fusion power could deliver electricity to the world’s grids within 15 years.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More on Linquip



