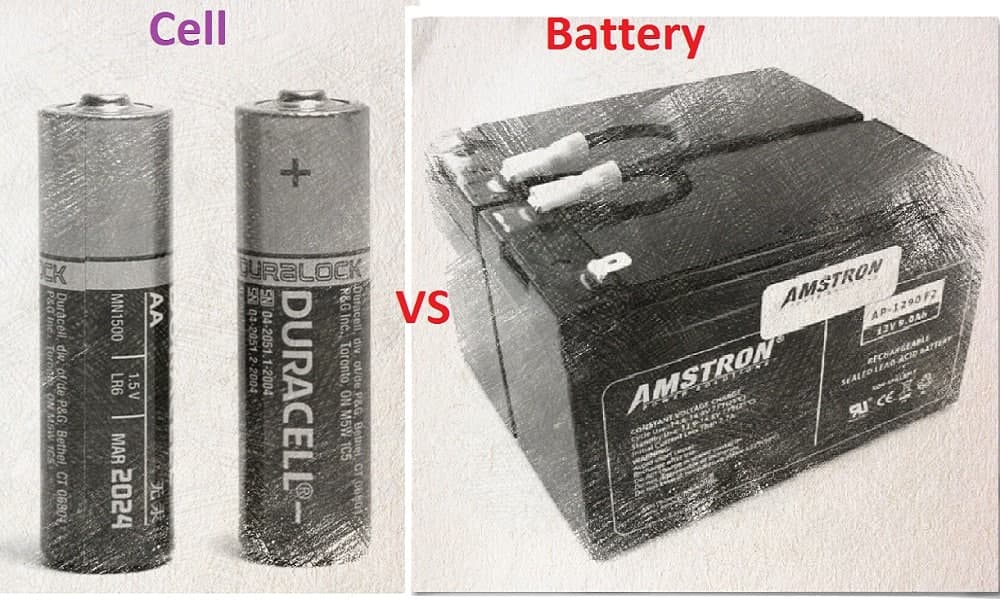
There are many types of power sources and cell and battery are direct voltage sources that produce a continuous direct voltage output. Cell and the battery are electrochemical devices that use a chemical reaction to generate electricity. They are both useful inventions that have made a lot of our daily tasks and life much easier. Cell and the battery practically find use in most of the portable electronic devices that we use today. Having said that, a cell and a battery are quite different from each other even though the terms are used interchangeably sometimes. In terms of how they are made and the functionalities, there are few significant differences between them. We are going to discuss the difference between a cell and a battery in the upcoming paragraphs. Read this new blog in Linquip to find out more about them.
What is a cell?
First, let us take a peek into the cells and batteries and how they help us keep up every single day before we dive into the differences between these two.
We encounter cells in all facets of our everyday lives. The importance of cells lies in their ability to provide us with a portable source of electrical energy. The cell is a single power generating unit that stores the chemical energy and then converts it into electrical energy. The cell has an electrolyte, a chemical substance that reacts with the electrodes and produces an electric current. It has two electrodes named the cathode and the anode. The cell does not lose energy over time if it is not in use. It is a practical way of storing energy, since it does not lose energy over time, which is why it is widely used, especially in a household appliance.
What is battery?
Battery is a source of energy power up most of the things we use every day. Its origin dates back to the late 19th century when Waldermar Jungner, a Swedish scientist, invented a battery with nickel, cadmium, and a calcium hydroxide electrolyte. By 1940 this battery began to be commercialized in the USA, and it was Thomas Alva Edison who created the model that is commercialized today based on the idea of Waldermar.
Battery is made up of electrochemical cells that have an electrolyte and two electrodes, one positive and the other negative (an anode and a cathode). They are also known as accumulators. Batteries lose their charge constantly, no matter if they are used or not. So, it needs continuous recharging so as not to lose its charge even if it is not in use. Batteries can store energy from solar and wind and discharge it when it is needed the most. They can have domestic and industrial use.
Cell vs. Battery
There is a description of the difference between cell and battery by considering several factors. Various factors, like those mentioned further, are taken into account while selecting a cell or a battery for a particular application.
The following factors show the main differences between cells and batteries.
Definition
- Cell converts chemical energy into electrical energy.
- Battery is the collection of electrochemical cells that either connects in series or in parallel.
Specification
- Cell has a single unit, and hence it is light and compact.
- Battery is a combination of cells that increase its size and make it bulky and heavy.
Power supply
- Cell supply power for a short period of time.
- Battery can supply power for long durations.
Applications
- Cell mostly finds use in clocks, lamp, radio, remote control devices, etc. that requires less energy.
- Battery is mostly used in automobiles, emergency lights, inverter, logistics and construction, military, etc.
Cost
- Cells are usually cheap.
- Batteries are much costlier.
Few other difference between cell and battery to keep in mind:
- Cell is a secondary generator, whereas the battery is a primary generator.
- Cell has one or more electrolytic cells, but batteries have a single electrolytic cell.
- Cell receives electrons from an external generator and stores them for later use, whereas the battery stores the potential to generate electrical energy through chemical energy.
- Cells are limited by the number of charges they can handle, but batteries are limited by their size.
So, there you have a detailed description of the difference between cell and battery. If you enjoyed this article in Linquip, let us know by leaving a reply in the comment section. Is there any question we can help you through? Feel free to sign up on our website to get the most professional advice from our experts.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Provider
Read More on Linquip
- Difference Between Energy and Power
- What Is Conduction Convection and Radiation? (With Example)
- What is the Difference Between Oscillator and Crystal?
- Difference between Electric and Magnetic Fields
- Distance Vs. Displacement: All You Should Know About
- What is Redox Flow Battery?
- Difference Between Mechanical and Electromagnetic Waves
- A Simple Guide to the Difference Between Motor and Generator
- Difference Between Hydraulics and Pneumatics
- Difference Between Current and Static Electricity
- Efficiency of Fuel Cell for Various Types of Fuel Cell
- 10 Best Solar Batteries for Residential and Commercial Use
- Top 22 Battery Suppliers & Manufacturers in USA & Worldwide (2022)