The main difference between density and relative density is how they are defined, where density is defined as the mass of a unit of volume, but relative density is defined as the density of a given substance divided by the density of a reference substance.
Basics of Density and Relative Density
Different suspensions in bodies of the same volume have varying masses. Density, which is the characteristic of each substance and depends upon mass and volume, determines physical size. A constant value is defined by dividing mass by volume as density.
According to the definition of relative density, it is the ratio of the density of that substance at a given temperature to a reference substance’s density at that same temperature or another temperature used as a reference.
Density vs. Relative Density in Terms of Definition
The density (mass per unit volume of a substance) is usually used in scientific work to describe the relationship between mass and volume. The industry is where relative density finds widespread application, generally due to historical reasons.
Density
In technical terms, density (more precisely, volumetric mass density, which is also known as specific mass) is the mass per unit volume of a substance. Density is usually represented by ρ. The density of matter can be defined mathematically as the mass divided by the volume:
\rho =\frac{m}{V}
ρ, m, and V are the density, mass, and volume respectively. It is sometimes, though scientifically inaccurately, defined as density as weight per volume (for example, in the oil and gas industry in the United States).
The density of a pure substance is the same as its mass concentration. Density varies widely among materials, and density is relevant to several properties, including buoyancy, purity, and packaging. At standard temperatures and pressures, osmium and iridium are the densest elements known to science.
Material density is affected by pressure and temperature. For solids and liquids, variation is typically small, but for gases, it is much greater. As pressure increases on an object, its volume decreases, and its density increases. When the temperature of a substance increases (with few exceptions), the volume of the substance increases, decreasing its density. Due to the decreased density of the heated fluid, heat convection usually occurs when heated at the bottom of a fluid.
Sometimes a substance’s reciprocal density is used, called the specific volume of the substance, which is related to thermodynamics. Density is an intensive property, meaning that increasing a substance’s amount does not increase the substance’s density; it instead increases its mass.
Here is a schematic of what is meant by the terms high-density, medium-density, and low-density.
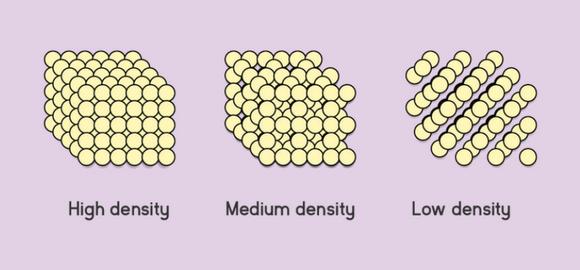
Relative Density
The relative density (RD) or specific gravity (SG) of a substance is a dimensionless quantity since it is the ratio of two values: densities or weights:
RD=\frac{{\rho }_{substance}}{{\rho }_{reference}}
Where ρsubstance represents the density of a substance being measured, and ρreference represents the density of a reference substance.
Unless the reference is explicitly specified, water at 4 °C (or, more accurately, 3.98 °C, which is the temperature when water reaches its maximum density) is typically assumed. As water has a density of approximately 1000 kg/m3, or 1 g/cm3, relative density calculations are made quite simple: the object’s density only needs to be divided by 1000 or 1, according to the unit.
The density of gases is often compared with the density of dry air at temperatures of 20 °C and pressures of 101.325 kPa, which has a density of 1.205 kg/m3. In order to find relative density with respect to air, you need to do the following:
RD=\frac{{\rho }_{gas}}{{\rho }_{air}}\approx \frac{M_{gas}}{M_{air}}
M stands for molar mass, and the sign approximately equal indicates that equality only pertains in scenarios where 1 mol of gas and 1 mol of air occupy the same volume at the same temperature and pressure, that is if both are Ideal gases. Normally, ideal behavior only occurs at very low pressures. For instance, a mol of an ideal gas occupies 22.414 liters at 0 °C and 1 atmosphere, whereas carbon dioxide occupies 22.259 liters at those same conditions.
The density of materials with a relative density (or an SG) greater than 1 is greater than that of water, so disregarding surface tension, they will sink in it. Those with relative densities less than 1 will float on the water since they are less dense than water.
Mathematically, true specific gravity is expressed as follows:
SG_{true}=\frac{{\rho }_{sample}}{{\rho }_{H_2O}}
The apparent specific gravity can be defined as the ratio of the weights of equal volumes of sample to water in air:
SG_{apparent}=\frac{W_{A,sample}}{W_{A,H_2O}}
Measurement of Density and Relative density
Besides the basic definitions, there are different ways to measure density and relative density.
Density
There are several methods and standards for measuring the density of materials. These techniques include:
- Hydrometer for liquids
- Hydrostatic balance for liquids and solids
- Immersed body method for liquids
- Pycnometer for liquids and solids
- Air comparison pycnometer for solids
- Oscillating densitometer for liquids
- Pour and tap for solids
There are different types of density being measured by each type of technique or method (e.g. bulk density, skeletal density, etc.), so a basic understanding of the type of density being calculated and the type of material in question is necessary.
Hydrostatic Pressure-based Instruments
This technology relies on Pascal’s Principle, which says that the pressure difference between two different points in a vertical column of fluid depends on the vertical distance between the two points, the fluid density, and the gravitational force. This technology is often applied for tank gaging purposes as a useful liquid level and density measure tool.
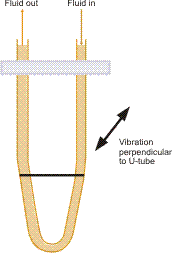
Vibrating Element Transducers
This type of device needs a vibrating element to be in contact with the fluid being measured. The element’s resonant frequency is measured and correlated to the fluid density with a characteristic dependent on the design of the element. In modern laboratories, accurate measurements of relative density are performed utilizing oscillating U-tube meters. These are able to measure 5 to 6 places beyond the decimal point and are applied in brewing, distillation, pharmaceutical, petroleum, and other industries.
Devices measure the actual fluid mass in a fixed volume at temperatures between 0 °C and 80 °C. However, because they are microprocessor-based, they can calculate apparent or true relative density and include tables relating these values to the strengths of acids, sugar solutions, etc.
Relative Density
In order to calculate relative density, simply measure the density of an object and divide it by a reference density. We know that a sample density is its mass divided by its volume. While mass is easy to calculate, the volume of a sample with an irregular shape may be more difficult to estimate.
Putting the sample into a graduated cylinder with water in it and reading what amount of water it removes is one method of testing. In an alternative method, the container can be filled to the brim with liquid, the sample immersed, and the overflow volume measured. There is a possibility that the surface tension of the water will keep a substantial amount from overflowing, which is problematic for small samples. It is therefore desirable to use a water container with a small opening.
Pycnometer
Pycnometers, also known as pyknometers or specific gravity bottles, are devices that measure the density of liquids. A pycnometer is made of glass and has a close-fitting ground glass stopper through which a capillary tube passes, enabling bubbles to escape from the apparatus. An analytical balance is used in this device to accurately measure a liquid’s density by referencing a suitable working fluid, such as water or mercury.

It is easy to calculate the relative density of a liquid by weighing the flask filled with water, empty, and full of any other liquid of the desired density. A pycnometer can also be used to determine the particle density of a powder, for which the usual method of weighing cannot be used. The powder is added to a pyrometer, which is weighed to determine the powder sample weight. A liquid of known density is filled into the pycnometer, in which the powder is completely insoluble. A relative density for the powder can be determined based on the weight of the displaced liquid.
By comparison, the gas pycnometer measures the pressure change caused by changing the volume of a closed container containing a reference (usually a steel sphere of known volume) with the pressure change due to the sample under the same conditions. This difference in pressure is indicative of the sample volume as compared to the reference sphere. This method is usually applied for solid particles that may dissolve in the liquid medium of the pycnometer design discussed above or for porous materials where the liquid might not fully penetrate.
Hydrostatic weighing
Without measuring volume, relative density can be measured easily and perhaps accurately. A spring scale is used to weigh the sample in air and then in water. Using the formula below, relative density (with reference to water) can be calculated:
RD=\frac{W_{air}}{W_{air}-W_{water}}
Wair represents the weight of the sample in the air (in newtons, pounds, or another force unit), and Wwater represents the weight of the sample in water (in the same units).
In this method, relative densities under 1 cannot be measured because the sample would float. Wwater becomes a negative value, representing the force required to keep the sample submerged.
There is another practical method that uses three measurements. A dry sample is weighed. Following the weighing of a full container of water, the sample is immersed in water and is weighed again after the displaced water is overflowed and removed. If you subtract the last reading from the sum of the two first readings, you will get the weight of the displaced water. Relative density is determined by dividing the dry sample weight by the displaced water weight.
Scales that cannot handle suspended samples can be used with this method. It is possible to handle a sample less dense than water, but it must be held down, and the errors entered by the fixing material must be taken into account.
Hydrometer
A hydrometer can be used to measure the relative density of a liquid. It includes a bulb affixed to a stalk with a constant area in the cross-section. See the example below:
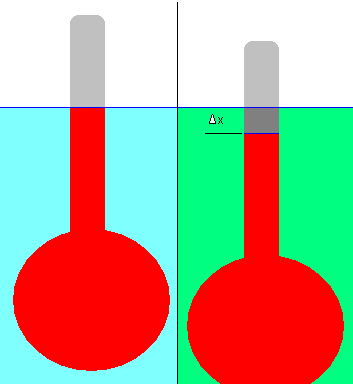
A hydrometer is floated in a reference liquid (shown in blue); then its displacement (the liquid level on the stalk) is marked (the blue line). Water is usually the reference fluid although it can be any liquid in practice.
After that, the hydrometer is floated in a liquid with an unknown density (green). Δx represents the displacement. Due to the slightly lower density of the green liquid than the reference liquid, the hydrometer has dropped slightly in this example. Hydrometers must float in both liquids, of course.
A change in displacement can be used to calculate the relative density of an unknown liquid using simple physical principles. To facilitate this measurement, the hydrometer’s stalk comes with graduation marks.
According to the Law of Equilibrium, the upward buoyancy force and the downward gravitational force acting on it must balance exactly. Gravitational force is simply the weight of the hydrometer. In accordance with Archimedes’ buoyancy theory, the buoyancy force acting on the hydrometer equals the weight of the liquid displaced. Multiplying the mass of displaced liquid by g gives this weight. By equating these, we obtain:
mg={\rho }_{ref}Vg
Or
m={\rho }_{ref}V*
Where m is the mass of the hydrometer, V is the volume of the displaced reference liquid (shown in red in the above figure), and ρref is the reference liquid’s density.
As for floating the hydrometer in the liquid being measured with a density of ρnew, the volume is:
V_{new}=V-A\mathit{\Delta}x
A is the shaft cross-sectional area. Thus,
m={\rho }_{new}(V-A\mathit{\Delta}x)
By combining the equations for the mass of the hydrometer, we will have,
RD=\frac{V}{V-A\mathit{\Delta}x}
On the other hand, according to the equation marked with *,
V=\frac{m}{{\rho }_{ref}}
Thus,
RD=\frac{1}{1-\frac{A\mathit{\Delta}x}{m}{\rho }_{ref}}
The relative density may be determined using this equation, which takes into account the displacement change, the density of the reference liquid, and the hydrometer’s known characteristics. If Δx is small, then the last equation may be represented as a first-order approximation of the geometric series equation:
RD\approx 1+\frac{A\mathit{\Delta}x}{m}{\rho }_{ref}
This illustrates that changes in displacement are roughly proportional to relative density changes for small Δx.



