Difference between Kinetic Energy and Potential Energy – The total forms of energy in a system can be categorized into two general types of macroscopic energy to conduct the work: potential energy and kinetic energy. Potential energy refers to the energy held by an object by virtue of its position, while kinetic energy is the energy possessed by an object due to its motion. Even though these main types of energy are very different, they are complementary. When potential energy is released, it always converts to kinetic energy, and kinetic energy is required for an item to store energy as potential in some form.
Kinetic Energy
What Is Kinetic Energy?
Kinetic energy, K, is a general type of energy that a particle or an object has due to its motion. When a net force is applied to an object and work is done, or in other expressions, the energy is transferred, the object accelerates and obtains kinetic energy. Kinetic energy is a feature of a moving item or particle that is determined by its mass as well as the motion achieved. Translation (movement along a path from one point to another), rotation around an axis, vibration, or any combination of motions are possible.
Kinetic energy can be shifted between objects and converted into other forms of energy, observing the law of conservation of energy. As an obvious example, the mechanical energy of the wind is converted into electrical energy by a wind turbine. The kinetic energy of a flowing fluid, in this example, air, is transformed into a rotating motion by a turbine. The wind rotates the wind turbine’s blades as it passes through them. These blades turn a generator which works as an inverse of an electric motor; instead of using electrical energy to spin and create mechanical energy, a generator uses mechanical energy to turn and produce electrical energy.

The joule (J) is the universally accepted unit of energy. A 2 kilogram (kg) mass moving at 1 meter per second (m/s) has one joule of kinetic energy. The newton-meter (N.m) and kilogram times meter squared per seconds squared (kg.m2/s2) are two other energy units. The erg, 10-7 joules, is the unit of energy in the centimeter-gram-second (CGS) system, and it corresponds to the kinetic energy of a flying mosquito. Other energy units, such as the even smaller unit, the electron volt on the atomic and subatomic scales, are employed in specific settings.
Kinetic energy is considered a scalar quantity; since it has no direction and is totally characterized by magnitude alone.
Translational Kinetic Energy
If a body mass m being pushed through a distance d along a surface by a force F parallel to that surface, the work W done would be:
W = \vec{F}\cdot \vec{d}= F\cdot d = m \cdot a\cdot d
With regard to the kinematic equations of motion, the acceleration a in the above equation can be substituted by the initial velocity (vi) and the final velocity (vf) and the distance:
W = m\cdot d \cdot \frac{v_{f}^{2}-v_{i}^{2}}{2d}= m \cdot \frac{v_{f}^{2}-v_{i}^{2}}{2}= \frac{1}{2}\cdot m\cdot v_{f}^{2}- \frac{1}{2}\cdot m\cdot v_{i}^{2}
Hence, by further simplifying the above equation, translational kinetic energy is given as follows:
K = \frac{1}{2} m v^{2}
This formula is valid only for low to relatively high speeds. However, when the object’s speed approaches that of the light, relativistic effects become prominent, and the relativistic formula is applied. If the object is atomic or subatomic in nature, the importance of quantum mechanical effects necessitates the employment of a quantum mechanical model.
Alternatively, the net work done on an object or system is equal to the change in kinetic energy:
W_{net}= \Delta K
Rotational Kinetic Energy
For a rotating body, the moment of inertia, I, corresponds to mass, and the angular velocity, ω, relates to translational velocity. Accordingly, rotational kinetic energy is computed as follows:
K = \frac{1}{2} I \omega ^{2}
Note that the total kinetic energy of a body or system is equal to the sum of the kinetic energies produced by each type of motion.
Types of Kinetic Energy
Different types of kinetic energy include radiant, thermal, sound, electrical, and mechanical ones. It’s worth mentioning that these types aren’t mutually exclusive; multiple kinetic energy examples can be observed in a single instance. To better understand these various types, let’s look at several kinetic energy examples below.
Radiant Energy
Radiant energy is the energy provided by electromagnetic waves or light and may be visible or invisible to the human eye. This energy, also known as electromagnetic energy, is most typically felt in the form of heat by humans. In general, anything that has warmth emits radiant energy. For example, as the heating elements of a toaster are warmed up, they emit radiant energy, which heats and toasts the bread.
Because kinetic energy is the energy of motion, radiant energy is ever in motion and travels across space or medium by waves or particles. Gamma rays, X-rays, ultraviolet light, visible light, infrared radiation, microwaves, and radio waves are all types of radiant energy.
Thermal Energy
Thermal energy is comparable to radiant energy in that both can be felt as heat or warmth. However, thermal energy represents the level of activity among the atoms and molecules in an object, whereas radiant energy refers to waves or particles. In other terms, thermal energy, often known as heat energy, is the internal energy of an object caused by the motion and collision of atoms and molecules. With our eyes, we can’t observe the mobility of this energy. When it contacts with our skin, though, we can feel it; such as hot spring or boiling water.
The kinetic energy of atoms and molecules determines an object’s thermal energy. The atoms in hotter objects move or vibrate quicker and have more kinetic energy. Hence, they will generate more thermal energy. Conversely, in colder objects, the atoms have less kinetic energy. As a result, they will produce less thermal energy.
Electrical Energy
The flow of negatively charged electrons in a circuit produces electrical energy, often known as electricity. When an external electric field or voltage is applied, the atom’s electrons gain energy and break their bonds with the parent atom, resulting in a free electron. This free electron’s energy is referred to as electrical energy. Some examples of electrical energy include lightning, batteries in use, desk lamp, etc.
Sound Energy
Sound energy is a type of energy created when an object vibrates. When an object vibrates, its energy is given to the surrounding particles, causing them to vibrate as well. These particles collide with other particles again, and so on. Likewise, sound energy is transferred from one particle to another. As a result, sound energy cannot move through a vacuum because there are no particles acting as a medium. It can only travel through a medium like water, air, and solid. Sound energy can be heard when it reaches a person’s ear. Alarms, vehicle horns, thunderstorms, etc., are all examples of sound energy.
Mechanical Energy
The energy correlated with the mechanical movement of an object is known as mechanical energy. The quicker an object moves, the more mechanical energy it possesses and the greater potential it has to perform work. Wind, a moving river, a bullet shot from a gun, fingers playing the piano, and so on are examples concerned with mechanical energy.
Potential Energy
What Is Potential Energy?
Kinetic energy can be stored. For example, it takes work to compress a spring. In this case, the kinetic energy is transmitted to potential energy and stored. Because, while it is not doing work, it has the potential to do so. The potential energy is returned into kinetic energy if we release the spring.
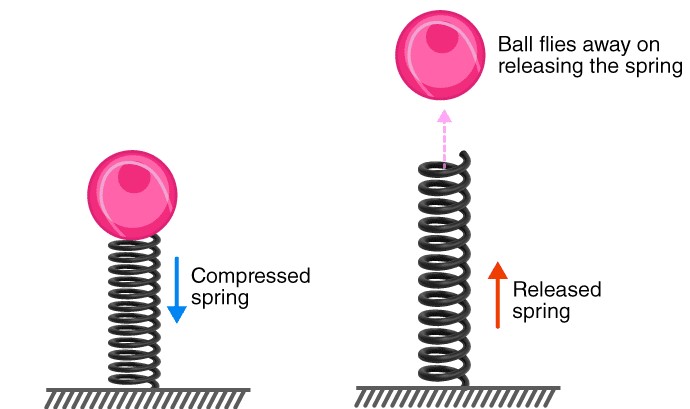
The potential energy, U, indicates the potential of an object to move and is typically defined as the stored energy held by a system due to the relative position of the parts subjected to a conservative force, which means the potential energy of a system of particles is determined only by its initial and final configurations, and it is independent of the path taken by the particles. In the case of throwing the ball to the top of the hill, regardless of the method or path via which the ball is elevated, the potential energy is the same if the ball’s initial position is ground level and the final position is 5 feet above the ground. Additionally, the value of potential energy is arbitrary and dependent on the reference point selected. In the preceding example, the system would have twice as much potential energy if the initial point was the bottom of a 5-foot deep hole.
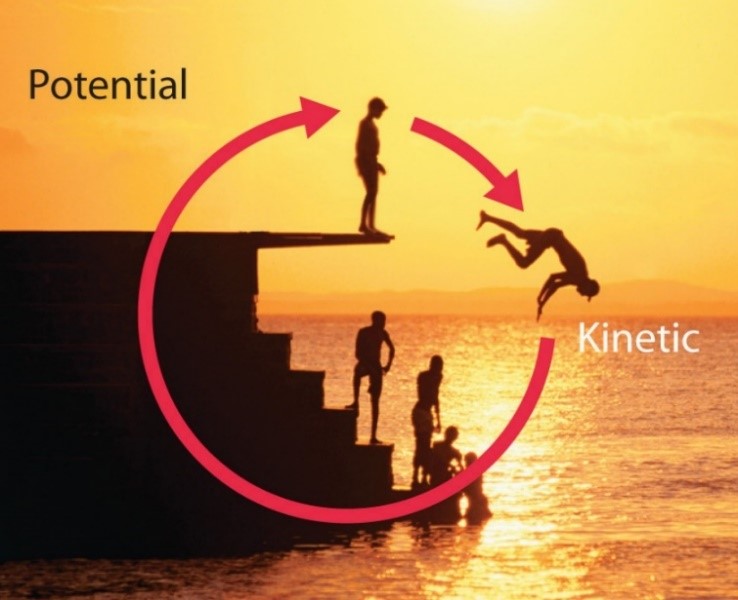
Potential energy can be converted to kinetic energy and vice versa. As the swimmer climbs the ladder to reach the diving board, the chemical energy is exchanged for mechanical work in order to overcome the force of gravity. When a swimmer stands at the end of a diving board, the potential energy is higher than before he ascended the ladder; the greater the distance from the pool, the higher the potential energy. As the swimmer steps off the platform to dive into the water, the potential energy is transformed into kinetic energy, and some of that energy is transferred to the water as the swimmer strikes the surface, leading it to splash into the air.
The units of potential energy are the same as those of kinetic energy: kg.m2/s2. It is measured using the SI unit joule (J) and is, therefore, a scalar quantity. If F represents the conservative force and x refers to the position, the potential energy of the object subjected to the force between the two positions x1 and x2 is expressed as the negative integral of F from x1 to x2:
U = -\int_{x1}^{x2}F(x)\cdot dx
Furthermore, the net work done on an object or system is equal to the change in potential energy:
W_{net}= \Delta U
Types of Potential Energy
Potential energy comes in a variety of forms, including:
- Gravitational potential energy
- Elastic potential energy
- Electric potential energy
- Nuclear energy
- Chemical energy
Gravitational potential energy
The amount of energy stored in an object as a result of its height above the ground within the gravitational field is called gravitational energy. The object will gain gravitational potential energy if work is done on it against gravity. For instance, when an object is lifted to a height, work is done against the gravitational force that pulls it down. As this lifted object is released from a height, its potential energy is turned back into kinetic energy.
As we know, the force needed to raise a body mass, m, is equal to m\times g . g is the gravitational field strength (9.81 on Earth). By substituting this value in the equation of potential energy, gravitational energy would be:
U_{grav} = m\times g\times h
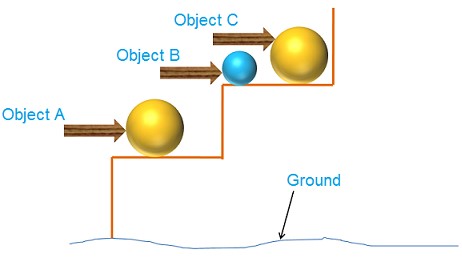
Hence, the amount of gravitational potential energy possessed by an object is determined by its mass, m, and height, h, above the earth’s surface as the reference point. As an example, consider the case of item C, which has more potential energy than object B due to its greater mass. Similarly, because item A is lower in height than object C, it has less potential energy.
Elastic potential energy
The energy stored in an object that is stretched or compressed is known as elastic energy. Elastic objects, such as springs and rubber bands, can store elastic potential energy. When you stretch a spring, the work or energy applied equals the quantity of elastic potential energy stored in the spring. The spring will turn back to its original shape if the force applied to it is withdrawn. At this point, as the equilibrium point, the amount of elastic energy stored in the spring is zero.
We already know that the force needed to stretch or compress a spring by x amount is given by k\times x , in which k is spring constant. With this regard, the elastic energy contained in the spring is expressed as:
U_{spring} = \frac{1}{2}k x^{2}
Electrical potential energy
Electric potential energy, also called electrostatic potential energy, is the stored energy that charged particles have due to its own electric charge and its relative position to other charged particles. As this energy results from conservative Coulomb forces, it can be calculated by:
F= k_{e} \frac{q_{1}q_{2}}{r^{2}}
k_{e}= \frac{1}{4\pi \varepsilon _{0}}
U_{E}=k\frac{q_{1}q_{2}}{r}
Where q1 and q2 are the values of the two charges, ke is the Coulomb constant in which ε0 is the permittivity of vacuum (= 8.85 ×10-12 C2N-1m-2 or epsilon naught value) and r is the distance between the charges.
Nuclear energy
Nuclear energy is the potential energy of particles found inside the nucleus of an atom, such as protons and neutrons. This energy is responsible for holding protons and neutrons together to create the nucleus. When two or more atomic nuclei join to produce a large nucleus, called nuclear fusion, or when one nucleus divides into two smaller nuclei, called nuclear fission, a great amount of nuclear energy is released as heat and light. The sun, for example, generates its energy by nuclear fusion of hydrogen into helium.
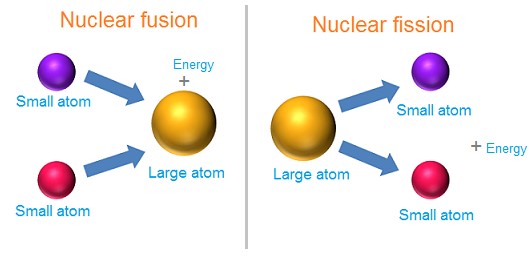
Nuclear particles are not destroyed in the process of nuclear fusion or fission since mass and energy are always conserved. The missing mass is transmitted to energy. In fusion, the combination of nuclear particles has less mass than if they were individually free, and this mass difference is released as heat and radiation in nuclear reactions. However, in the fission process, a large atomic nucleus is split and some of the mass is given off as energy.
Chemical energy
The energy held in the chemical bonds of atoms and molecules is referred to as chemical potential energy. When the bonds between the atoms are broken, chemical energy is frequently released in the form of heat. Batteries, wood, coal, and gasoline are all examples of chemical energy. As an example, when gasoline is burned in a car’s engine, it contains a substantial amount of chemical energy that is released. Some of this released energy is converted into work and used to move the car forward. Simultaneously, some of that is converted to heat, causing the car’s engine to become hot.
Difference between Kinetic Energy and Potential Energy
The primary difference between potential and kinetic energy is that one is the energy of what can be, while the other is the energy of what already is. In other terms, potential energy is the energy that is stored and ready to be released, whereas kinetic energy is energy that is being used for movement. Another significant distinction is velocity. This is the foundation of kinetic energy, yet it has nothing to do with potential energy. When calculating the amount of kinetic energy for any given object, velocity is the most significant portion of the equation, yet it is absent from the potential energy equation. In the context above, the formula for estimating the amount of this energy in various forms is mentioned.
Some other differences that are significant between kinetic energy and potential energy are explained as below:
- Nature: The energy possessed by an object as a result of its motion is known as kinetic energy. In comparison, potential energy is the energy that an object has as a result of its position.
- Transformation: It is possible for kinetic energy to be transferred from one item to another through collisions. But the potential energy cannot be transmitted from one item to another.
- Symbol: Kinetic energy is denoted by the symbol K, or sometimes KE, whereas potential energy is expressed by U or PE.
- Measurement: The measurement of Kinetic Energy takes place at the location where the object is at the time of measurement. For example, if a ball is moving upwards at 50 m/s and we need to measure the Kinetic energy at the height of 3 meters above the ground, the measurement is made at that location rather than with reference to another place. When measuring potential energy, the measurement is made by subtracting the current position from the reference position. The potential energy of a ball that is 10 meters above the ground and at rest is measured using the ground as a reference.
- Environment: Kinetic Energy is affected by the environment and is dependent on it. While the environment, on the other hand, has no effect on potential energy.
You are also encouraged to visit the Liqnuip website to find out more information about various equipment and tools related to kinetic and potential energies.
Read More on Linquip



