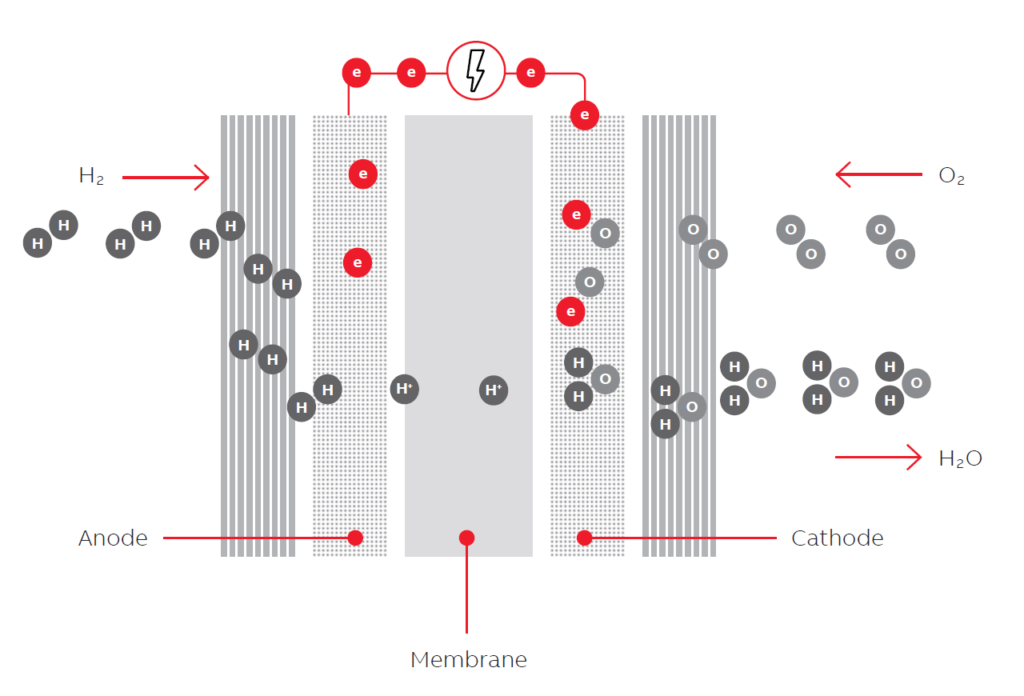
The efficiency of fuel cell_ A fuel cell is a device that produces electricity by a chemical reaction. All fuel cells have two electrodes called the anode and cathode. The reactions that generate electricity occur at the electrodes.
Each fuel cell also has an electrolyte, which offers electrically charged particles and a catalyst that speeds the reactions at the electrodes.
However, if waste heat is recovered in a cogeneration scheme, efficiencies of up to 85% can be achieved. The energy efficiency of a Fuel Cell is typically between 40 and 60 percent. Several Suppliers and Companies, as well as various manufacturers and distributors, provide various types of fuel cells, and there are several types of Fuel Cells for Sale on Linquip.
There is a comprehensive list of services on the Linquip website that covers all factory operations. Linquip vendors can assist you with this. Please contact Fuel Cell Experts in Linquip to learn more about how to connect with a diverse group of service providers who consistently deliver high-quality products.
Although less efficient compared to electric batteries, hydrogen fuel cells compare favorably with internal combustion engine technology, which extracts kinetic energy from the fuel at approximately 25 percent efficiency. A fuel cell, by contrast, can process hydrogen with air to generate electricity at up to 60 percent efficiency.
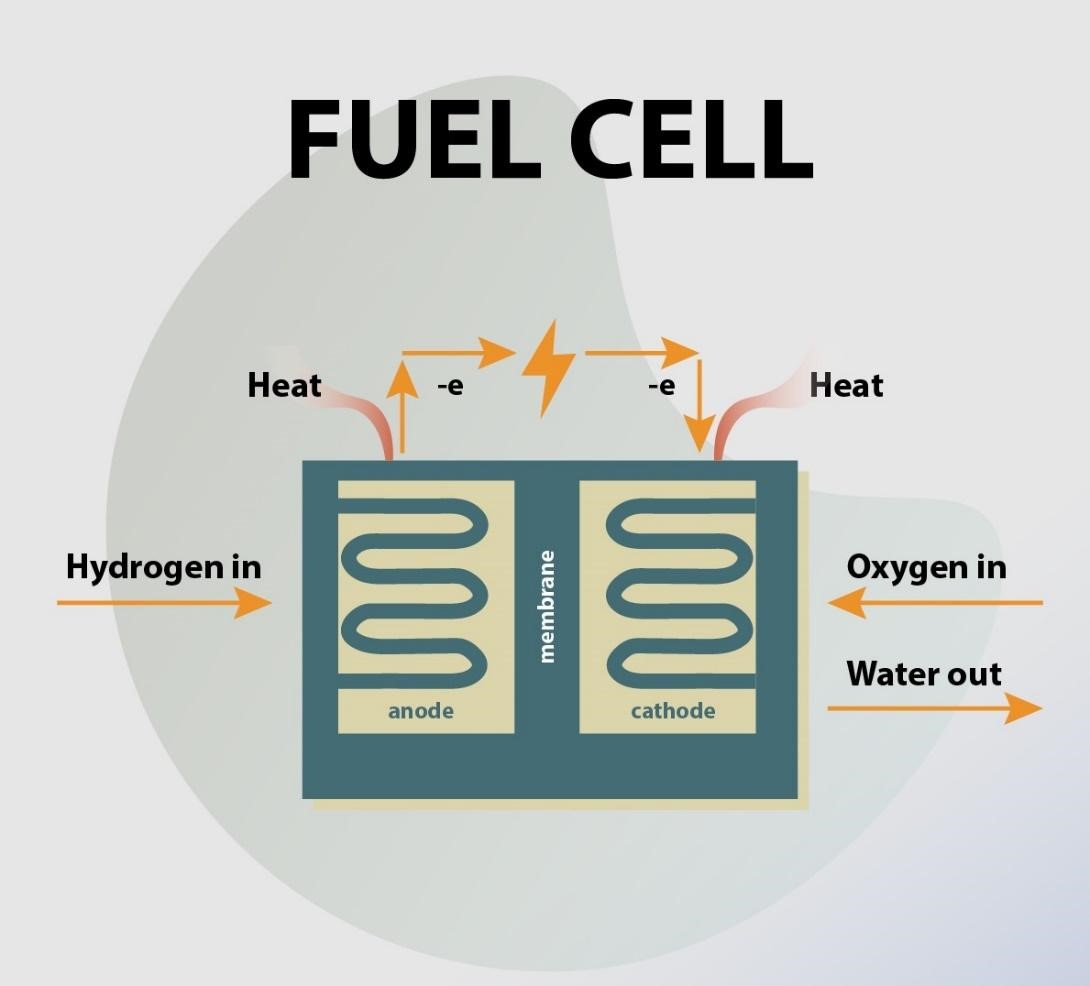
Types of Fuel Cell
Each fuel cell has two electrodes, an anode and cathode, and an electrolyte connecting them. Fuel cells have various electrolytes and electrodes, and their electrochemical processes occur at different temperature levels. As such, each kind of fuel cell (or fuel cell technology) has its inherent pros and cons, making them more proper for specific markets and applications.
Alkaline Fuel Cell
Alkaline fuel cells (AFCs) were revealed in 1959 by Francis Thomas Bacon. They have a liquid alkaline electrolyte such as potassium hydroxide (KOH) in water and cathodes that are regularly made with platinum. Performing at 60-70oC (140-158oF), AFCs are one of the most efficient fuel cells that can reach up to 60% efficiency and approximately 87% combined heat and power.
Spaceships in the US and Russian/Soviet used alkaline fuel cells to provide electricity and drinking water for astronauts. Other advantages of AFCs are their virtually instant performance without pre-heating, even at sub-zero temperatures, and their resistance to salt air and humidity. Also, AFCs are used as long-duration UPSs or backup generators for powering telecom towers and urban buses.
Proton Exchange Membrane Fuel Cell
Proton exchange membrane fuel cells (PEM or PEMFC fuel cells) use platinum group-based electrodes and a water-based or mineral-acid-based polymer membrane as an electrolyte. The water-based fuel cells function at 80-100oC (176-212oF), and the mineral-acid-based PEMs, identified as high-temperature PEMs (or HTPEMs), function at up to 200oC (224oF). They need precise humidity conditions to work, and their acidic nature demands a platinum catalyst.
PEM fuel cells are comparatively small and light-weight and are consequently the leading fuel cell technology applied in material handling applications such as forklifts and transportation applications, including buses, cars, and trucks. PEMFCs are the fastest-growing variety of fuel cells.
Phosphoric Acid Fuel Cell
This type was developed in the mid-1960s and field-tested in the 1970s; the Phosphoric acid fuel cells (PAFCs) are one of the most mature fuel cells and the first model to be commercially accepted. PAFCs use phosphoric acid as an electrolyte and an anode and cathode composed of a finely dispersed platinum catalyst on silicon carbide and carbon structure. Typically, they have been utilized for stationary power generation in buildings, hospitals, hotels, and utilities in the USA, Europe, and Asia.
These systems have been technically prosperous and very reliable, with 40% plus efficiency levels and tens of thousands of working hours. Water management in this type is more straightforward than in PEMs, and they are more tolerant of contaminants in hydrogen. However, the emission of phosphoric acid is doubtful, and good ventilation is necessary. PAFCs are less powerful than other types in the same weight and volume and need much more platinum than other fuel cells, which increases their cost.
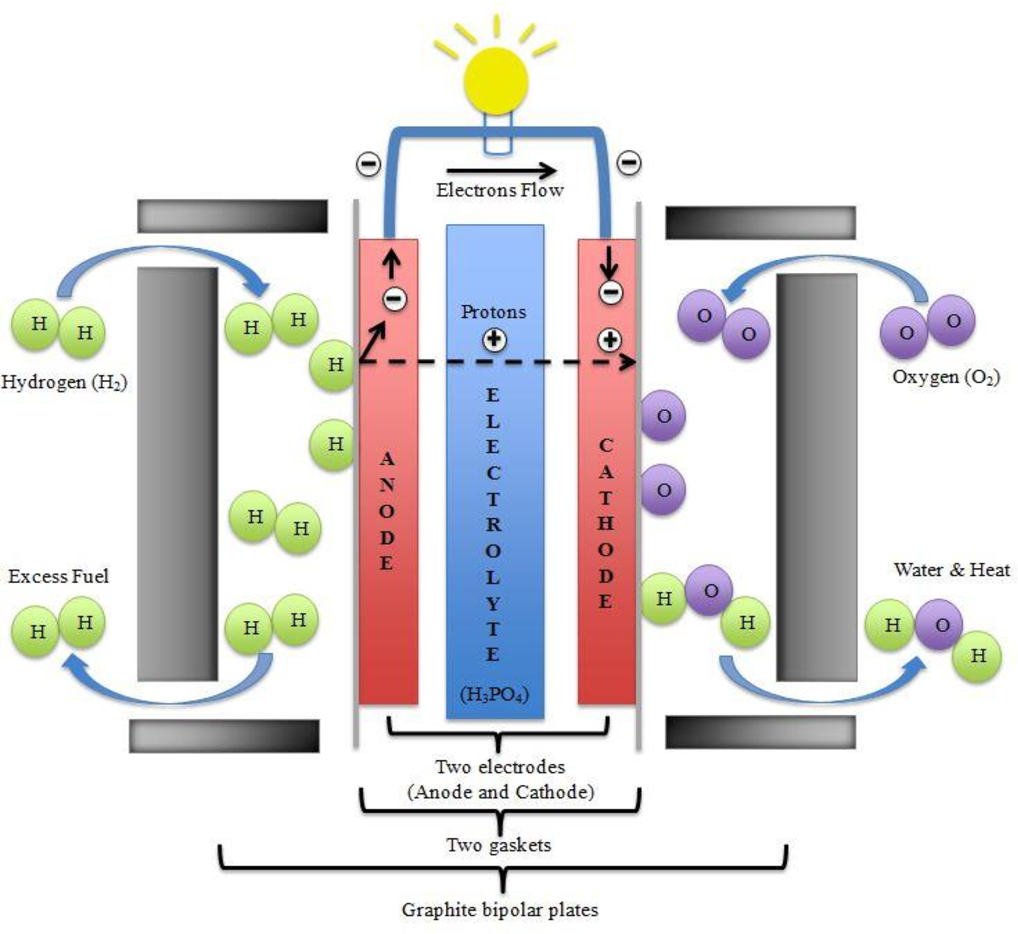
Molten Carbonate Fuel Cells
This type uses a molten carbonate electrolyte and operates at 650 oC, enabling them to function on unreformed fuels such as methanol, ethanol, biogas, natural gas, and coal. In addition, the absence of a catalyst produced from noble metals such as gold, silver, or platinum makes MCFCs be more cost-competitive with more conventional sources of power. The efficiency of fuel cells in MCFCs is close to 50%, increasing up to 80% when high-quality waste heat is reabsorbed.
MCFCs need a large number of stainless steel and nickel parts that raise the materials cost, requiring specialized manufacturing techniques. Molten carbonate is also naturally corrosive. As the running temperature is so high, MCFCs need significant time to touch operating temperature and are slow in responding to sudden changes in electricity requirements. However, they are best suited for the preparation of constant power in large utilities.
Solid Oxide Fuel Cells
Solid oxide fuel cells are manufactured of a thin layer of ceramics. The ceramics in SOFCs are not electrically and ionically active until they are in the range of 500-1000 oC (1060-2120 oF). The high temperature enables them to oxidize almost any fuel, including gasoline, natural gas, diesel, biofuels, hydrogen, and even coal gas. The ceramic construction for providing stability and reliability makes SOFCs more costly than other fuel cells. The solid electrolyte is constructed from a ceramic material named Yttria-Stabilized Zirconia (YSZ). Considering the high running temperature, SOFCs need significant time to reach working temperature and are slow to respond to changes in electricity requirements.
The table below presents the specification of the described fuel cells. (Reference: gencellenergy.com)
| FC Type | Proton Exchange (PEM & HTPEM) |
Molten Carbonate (MCFC) | Alkaline (AFC) |
Phosphoric Acid (PAFC) | Solid Oxide (SOFC) |
| Anode | Platinum | Steel/nickel | Platinum or Carbon (GenCell) | Platinum | Ceramic |
| Electrolyte | Polymer Membrane | Molten Carbonate | Potassium Hydroxide (KOH) | Phosphoric Acid (H3PO4) | Yttria-Stabilized Zirconia (YSZ) |
| Electrolyte Type |
Solid | Solid | Liquid | Liquid | Solid |
| Fuel | • Hydrogen | • Natural gas • Methanol • Ethanol • Biogas • Coal gas |
• Hydrogen • Ammonia (GenCell) |
• Hydrogen • Methanol |
• Natural gas • Methanol • Ethanol • Biogas • Coal gas |
| Temperature | • 80-100 ºC (176-212 ºF) • 200 ºC (224 ºF) |
• 650 ºC (1202 ºF) |
• 60-70 ºC (140-158 ºF) |
• 150-200 ºC (336-448 ºF) |
• 500-1000 ºC (1060-2120 ºF) |
| Efficiency | 30-40% | 50% (80% CHP) |
60-70% (80% CHP) |
40-50% (80% CHP) |
60% |
| Power | 0.12-5 kW | 10 kW – 2 MW | 0.5–200 kW | 100 – 400 kW | 0.01 – 2000 kW |
| Startup Time | < 1 minute | 10 minutes | < 1 minute | n/a | 60 minutes |
| Pros | • Quick startup • Small • Light-weight |
• Fuel variety • Efficient |
• Quick startup • Temperature resistant • Low-cost ammonia liquid fuel |
• Stable • Maturity |
• Fuel variety |
| Cons | • Sensitivity to humidity or dryness • Sensitivity to salinity • Sensitivity to low temperatures |
• Slow to respond • Highly corrosive |
• Liquid catalyst adds weight • Relatively large |
• Phosphoric acid vapor • Less powerful |
• Long startup time • Intense heat |
| applications | • Cars • Buses • Trucks |
• Utilities | • Backup generators (long-duration UPS) • Primary power generators • Off-grid telecom |
• Buildings • Hotels • Hospitals • Utilities |
• Corporate power plants |
Direct Methanol Fuel Cells
Most fuel cells operate with hydrogen, which can be supplied to the fuel cell system directly or can be provided within the fuel cell system by converting hydrogen-rich fuels such as methanol, ethanol, and hydrocarbon fuels. However, Direct Methanol Fuel Cells (DMFCs) are powered by pure methanol, which is regularly mixed with water and directly fed to the fuel cell anode.
Direct methanol fuel cells do not have fuel storage problems typical of some fuel cell systems as methanol has a higher energy density compared to hydrogen, though less than diesel fuel or gasoline. Methanol is also simpler to transport and supply using modern infrastructure because it is a liquid, similar to gasoline. DMFCs are often used to produce power for portable applications, the same as cell phones or laptop computers.
Reversible Fuel Cells
Reversible fuel cells generate electricity from hydrogen and oxygen and produce heat and water as byproducts, similar to other fuel cells. However, reversible fuel cell systems can also utilize electricity from wind power, solar power, or other sources to break water into oxygen and hydrogen fuel by electrolysis process. Reversible fuel cells can produce energy when needed, and during the high power generation from other technologies, reversible fuel cells can save the excess energy in the shape of hydrogen. This energy storage capacity could be a key enabler for alternate renewable energy technologies.
The Efficiency of Fuel Cells
The efficiency of fuel cells can be named as theoretical and practical, which vary in terms of action and equations.
Theoretical Maximum Efficiency of Fuel Cel
The energy efficiency of a system or method that converts energy is measured by the ratio of the amount of helpful energy (“output energy”) to the entire amount of energy (“input energy”) or by valuable output energy as a percentage of the whole input energy. In fuel cells, the output energy is electrical energy generated by the system. The input energy is the stored energy in the fuel.
According to the US Department of Energy, the efficiency of fuel cells is generally between 40 and 60%. It is much higher compared to some other systems in energy generation. For instance, the typical internal combustion engine of a car is around 25% efficient. In Combined Heat and Power (CHP) systems, the heat delivered by the fuel cell is obtained, increasing the system’s efficiency to up to 85–90%.
The theoretical maximum efficiency of power generation systems never reaches in practice. However, this calculation provides a comparison of various kinds of power generation systems. The theoretical maximum efficiency of a fuel cell approximates 100%, while this efficiency for internal combustion engines is roughly 58%.
The efficiency of a Fuel Cell in Practice
The practical efficiency of a fuel cell is less than the theoretical one and varies from case to case. In a fuel cell vehicle type, the tank-to-wheel efficiency is higher than 45% at low loads and presents average rates of about 36% when a driving cycle similar to the NEDC (New European Driving Cycle) is applied as a test procedure. The comparable NEDC efficiency for a Diesel vehicle is about 22%. In 2008 Honda published a fuel cell electric vehicle (the Honda FCX Clarity) with a fuel stack declaring a 60% tank-to-wheel efficiency.
Fuel cell vehicles operating on compressed hydrogen may have nearly a power-plant-to-wheel efficiency of 22% if the hydrogen is deposited as high-pressure gas and 17% if it is deposited as liquid hydrogen. Fuel cells can not save energy similar to a battery, except hydrogen. Still, in some utilization, such as stand-alone power plants based on discontinuous sources such as solar or wind power, they are coupled with electrolyzers and storage arrangements to form an energy storage scheme.
Since 2019, 90% of hydrogen was utilized for oil refining, chemicals, and fertilizer production, and almost 98% of hydrogen is provided by steam methane reforming, which releases carbon dioxide. The entire efficiency (electricity to hydrogen and reverse to electricity) of these plants (identified as round-trip efficiency), utilizing pure hydrogen and pure oxygen, can be in the range of 35 to 50 percent, based on gas density and other provisions. The electrolyzer/fuel cell system can deposit unlimited quantities of hydrogen and is suited for long-term storage.
Solid-oxide fuel cells generate heat from the recombination of oxygen and hydrogen. The ceramic types can operate in 800 degrees Celsius. This heat can be recovered to be used in the system to increase the efficiency of fuel cells.
Calculating Fuel Cell System Efficiency
In a fuel cell reaction, the total energy is composed of both electrical and thermal energy identified as enthalpy (H). The Electrical energy is associated with the Gibbs free energy (G) and corresponds to the maximum usable electrical energy obtainable when hydrogen recombines with oxygen.
Irreversible energy in the system or entropy (S) is the “cost of doing business” and depends on the reaction’s temperature. The loss due to entropy is comparable with a bouncing ball losing energy when hitting the floor, as friction from the bouncing action creates a transfer of thermal energy to atoms in the bottom. The energy conveyed to those floor atoms dissipates and can not be recovered. Consequently, the change (Δ) in certain quantities from a standard set of conditions follows the equation shown below:
\Delta H=\Delta G+T\Delta S
The conventional method for calculating the efficiency of fuel cell power plants or other electrical generation devices is to divide the electricity generated by the Higher Heating Value (HHV) of the fuel employed. This is a reasonable way for calculating power plant efficiency because the power plant supervisor purchases fuel (natural gas is traded by heating value) and sells electricity.
Electrical Efficiency= (Electricity Produced)/(HHV of Fuel Used)
Accordingly, there is a maximum theoretical limit for the electrical efficiency achievable by a fuel cell system expressed by the Gibbs free energy divided by the heat of combustion of the fuel.
For the hydrogen fuel cell, this value is:
Gibbs free energy/HHV = 237.2 kJ/mole ∕ 285.8 kJ/mol= 83%
The use of HHV here is in conformity with the method used in the United States to determine efficiency for internal combustion (IC) generators/engine and gas generator/turbine systems. However, some US developers of high-temperature fuel cells use the European convention and hydrogen’s lower heating value (LHV) for efficiency calculation.
The maximum theoretical efficiency of a whole fuel cell system based on LHV of hydrogen is 94.5% or 228.6 kJ/mole ∕ 241.8 kJ/mol. As it is clear, using the LHV convention for measuring the efficiency of an electrical generator always generates numbers greater than those yielded by calculations employing the HHV for the same system. When requesting the electrical efficiency of an electric generator, it is essential to indicate whether it is based on the LHV or HHV calculation method.
Fuel cells can not reach these maximum electrical efficiency numbers due to internal resistance losses. For instance, a practical fuel cell working near its maximum power output might produce only 154 kJ of electricity per mole of hydrogen consumed. The rest of the heating value appears as heat produced by the fuel cell. The calculation for such a fuel cell is presented as 154 kJ/mol∕ 285.8 kJ/mol= 54% efficient (HHV) in the practical process. 46% of the energy generated can be recovered from the fuel cell as co-generated heat.

Voltage Efficiency of Fuel Cells and Stacks
The electrical system efficiency considerations discussed above are employed globally to whole fuel cell systems that involve many individual components, such as humidifiers, fuel processors, fuel cell stacks, power conditioners, and controls. Many experimenters and developers wish to evaluate the efficiency of the fuel cell stack separate from the system’s efficiency.
For this reason, it is helpful to use the theory of voltage efficiency, which is described as the cell (or cell stack) operating voltage divided by the thermodynamic cell voltage.
Voltage Efficiency= (Operating Voltage (V))/(Thermodynamic Voltage (E))
The thermodynamic cell voltage can be determined using the Nernst equation and the Gibbs free energy:
E=E^{0}-\frac{RT}{nF}ln\frac{[H_{2}O]}{[O_{2}]^{1/2}[H_{2}]}
Where:
- E° = the thermodynamic voltage under standard conditions
- E = the thermodynamic voltage under prevailing conditions
- n = the number of electrons transferred (2 in this case)
- F = Faraday constant (96,485 Coulomb mole-1)
- R = the gas constant (8.314 J deg K-1 mole-1)
- T = temperature in degrees Kelvin (25°C = 298 K)
- [ ] = the thermodynamic activity of the products and reactants. For gases, it can be approximated by partial pressure in the atmosphere .
As shown below, Gibbs free energy can be transformed into a thermodynamic voltage applying the formula as free energy is in joules per mole:
Gibbs free energy (in standard conditions) = nFE°
The Gibbs free energy for the hydrogen-oxygen reaction, under standard conditions, is 237.2 kJ/mol for the result of liquid water at 25°C. Consequently, the thermodynamic voltage for a hydrogen-oxygen fuel cell working at standard temperature and pressure is 1.229 volts.
E^{0}= \frac{237200J}{2(96485)J}=1.229 volts
The Nernst equation is helpful for calculating the thermodynamic voltage at alternating pressures and reactant concentrations. Still, it should be regarded that the Gibbs free energy of the hydrogen/oxygen reaction varies with temperature. To calculate the thermodynamic voltage of a cell at another temperature, it is required to find the Gibbs free energy for the reaction at that temperature in thermodynamic tables. For instance, the thermodynamic voltage is 0.998 volts for a high-temperature fuel cell running on hydrogen/oxygen at atmospheric pressure and 1,000K.
Experimenters and developers of higher-temperature fuel cells regularly use the free energy to form water vapor rather than liquid water in their calculations. We can find both in most thermodynamic tables. A suitable fuel cell with well-sealed parts and a properly operating electrolyte should display a voltage close to the thermodynamic voltage when it is not generating power (no-load).
This is also recognized as the open-circuit voltage. Pinholes in the electrolyte that allow fuel and oxidant to process, for example, decrease the open-circuit voltage and indicate a problem. The approximate efficiency for a fuel cell stack that is generating electrical power can be determined by dividing the operating voltage by the thermodynamic voltage. Therefore, a polymer electrolyte membrane (PEM) fuel cell running at 0.800 volts under standard conditions has a 0.800 V ∕ 1.229 V = 65% voltage efficiency.
Download Efficiency of Fuel Cell PDF
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More In Linquip
- Efficiency of Inverter
- Electric Heater Efficiency and Running Costs
- Space Heater Efficiency For Various Types of Heaters
- Heater Efficiency Calculation: Formula & Equation
- Fan Efficiency Calculation: Formula & Equation
- Efficiency of Wind Turbines
- DC Motor Efficiency: Calculation: Formula & Equation
- Efficiency of Diesel Generators Calculation: Formula & Equation
- Energy Efficient Electric Heater: A Practical Guide
- What is Generator Efficiency? Calculation & Formula Guide
- Efficiency of Induction Motor: Calculation & Equation
- Difference Between Cell and Battery: Ultimate Guide