The main difference between electromagnetic waves and matter waves is in the components that make them. There are three types of waves, of which electromagnet wave and matter wave are two. In this article difference between electromagnetic waves and matter waves is explained.
The wave is perceived as a disturbance if there are constant oscillations of components at a particular spot or in close areas. In this way, a wave transmits the information or the energy to be scattered from a source to a receiver without the physical transfer of materials.
Waves are separated based on their characteristics. The main characteristics include the orientation, direction, and motion of the particles. Based on this, waves are classified into three categories: mechanical waves, electromagnet waves, and matter waves.
What Is Wave?
In physics and mathematics, a wave refers to a propagating dynamic disturbance that deviates from equilibrium. In many cases, a wave can be described by the wave equation. Physical waves are created by the involvement of at least two quantities in the wave environment.
In the case of a periodic wave, this quantity fluctuates frequently around the amount of equilibrium, i.e. the rest position. A wave that moves in one direction is called a traveling wave. But two waves superimposed in opposite directions form a standing wave.
Mechanical and electromagnetic waves, as the two main categories of waves, are able to move energy, momentum, and data, but not the particles.
However, standing waves, as a base of music sounds and hydraulic jumps do not have motion. Also, some waves such as the quantum probability waves are overall stationery.
The highest point of a wave is called the wave crest and the lowest point is called the wave trough.
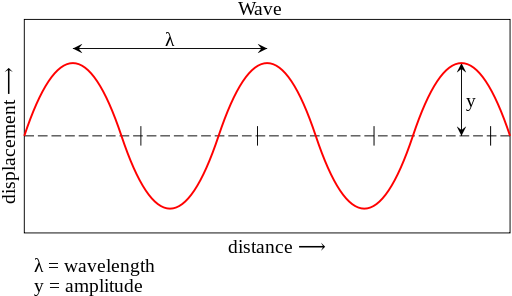
In the following image, you can see the primary shape of waves and their basic characteristics.
What Is Wavelength?
Wavelength is referred to the distance between two consecutive peaks or two similar consecutive points in a wave. Wavelength is measured in length units like meters.
What Is Frequency?
In physics, frequency is defined as the number of waves that pass through a point in the unit of time. The frequency of a wave is measured by the unit of Hertz (Hz).
The simplest equation to calculate the frequency is given as:
f=\frac{1}{T}
T denotes the time for a wave to complete a cycle in seconds.
What Is Amplitude?
The maximum amount of displacement of a point of a wave relative to its rest position (equilibrium) is called amplitude. So the amplitude is half of the vibration length. Like wavelength, it is measured in length units.
What Is Electromagnetic Wave?
An electromagnetic wave is the result of oscillation between electric and magnetic fields. In other words, it is created by vibrating magnetic and electric fields. It is also called the EM wave.
An electric field is created by electrically charged particles. This electric field accelerates the particles. Charged particles themselves generate a magnetic field that exerts a force on the particles. Therefore, these excitations can be considered as the result that changes in the magnetic field cause changes in the electric field and vice versa.
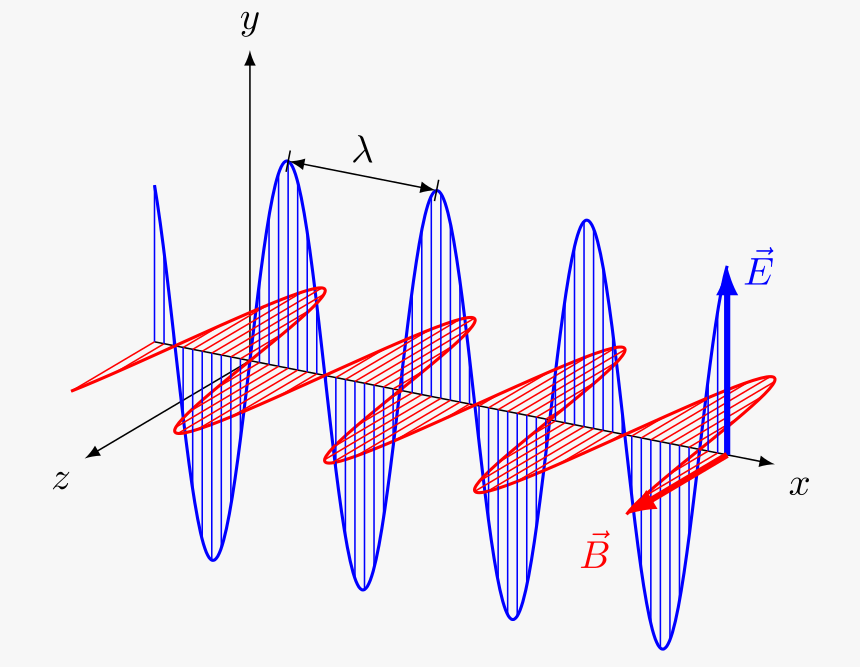
The following figure shows an example of an electromagnetic field with an electric and magnetic field perpendicular to each other. Also, the propagated wave spreads in a direction perpendicular to these two axes.
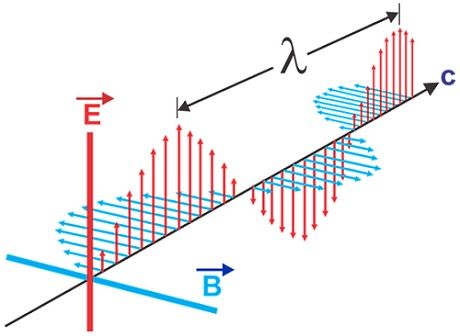
Electromagnetic waves do not need a medium to propagate, unlike mechanical waves that necessarily need a medium to travel. They are spread at the speed of light (300,000,000 meters per second) in a vacuum. The speed of the electromagnetic wave is denoted by c.
These waves are measured with amplitude and wavelength characteristics, so they are transverse waves.
Electromagnetic Radiation
Radiation is the energy that moves and spreads. In physics, electromagnetic radiation is what the waves of the electromagnetic field do to propagate in the space and transfer the electromagnetic radiant energy. Electromagnetic radiation includes synchronized vibrations of magnetic and electric fields.
In an isotropic medium, the axes of oscillations of the electric and magnetic fields and also the wave propagation direction are all perpendicular to each other to form a transverse wave.
The electromagnetic wave front emitted from a point source is a sphere. The position of an electromagnetic wave in the spectrum of these waves is determined based on its wavelength and frequency.
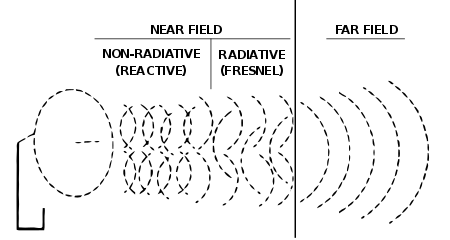
Electromagnetic radiation generally belongs to those electromagnetic waves that are free from being affected by excitatory charges, in which case they are so-called far fields. Near fields are those that refer to currents and their generating charges, such as electromagnetic induction and electrostatic induction.
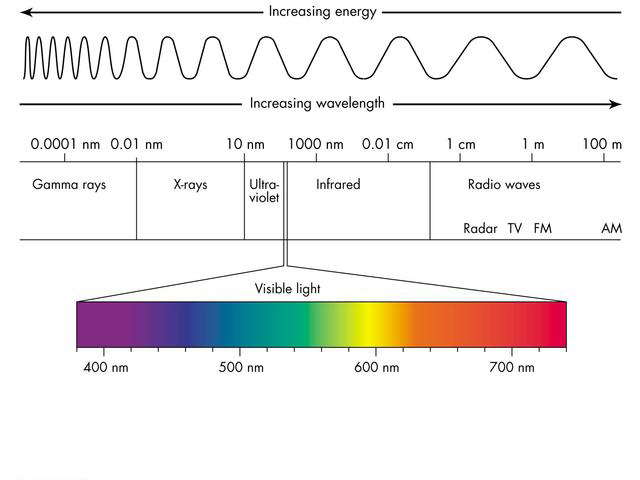
Electromagnetic Spectrum
The range of radiation of all electromagnetic waves is called the electromagnetic spectrum. Electromagnetic waves based on decreasing the wavelength and increasing the frequency are:
Radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
The effect of electromagnetic waves on chemicals and organisms depends on the strength and frequency of the radiation. The radiation of visible light and other EM waves of lower frequency ranges are in the class of non-ionizing radiation because of their insufficient photons energy to ionize atoms.
The first effect of electromagnetic radiation on organisms and chemicals is due to the heat, which is caused by a large number of transmitted photons.
Contrarily, radiations with higher frequencies than visible light like ultraviolet and X-rays are ionizing radiations due to the great energies of individual photons to ionize molecules. These radiations can initiate chemical reactions. Also, they can destroy living tissues in addition to heating. This is the cause of considering these high-frequency radiations as a health hazard.
Radio Waves
Radio waves with long wavelengths (30 centimeters to several thousand meters) are propagated by radio stations and reach our radio equipment.
Microwave
Microwave radiation with a smaller wavelength than radio waves (3 millimeters to 30 centimeters) is used to cook and prepare food, as well as to obtain information about the structure of galaxies by astronomers.
Infrared Radiation
Infrared radiation with a wavelength range of (700 nanometers to 1 millimeter) is used for applications such as capturing light from objects and skin with night vision goggles with heat.
Visible Light
Human eyes see visible light in the wavelength range of 380 to 700 nanometers.
Ultraviolet Radiation
Every hot object like the Sun emits ultraviolet radiation with a wavelength range of 100 to 400 nanometers. These waves are the cause of many skin diseases among people.
X-Ray
X-rays are used for dental photography or airport security use to check the contents of a travelers’ bag. Their wavelength range is 10 picometers to 10 nanometers.
Gamma Ray
Gamma waves are used for common medical imaging to see inside the body. Their wavelength is less than 10 picometers.
In the following figure, the electromagnetic spectrum, the range of each wave type, and the changes in energies and wavelengths are shown.
What Is Matter Wave?
Matter waves are the result of the motion of basic particles, including protons, neutrons, and electrons, along with molecules and atoms. The naming of these waves is that they deal with the matter.
These waves form the basis of quantum theory, which is a pattern of particle-wave duality. The electron beam is scattered just like a beam of light. But because of the small wavelength, it does not have a significant impact on our daily lives. Therefore, matter waves do not play a significant role in our ordinary affairs due to their too small wavelengths.
For the first time in 1924 Louis de Broglie, the French physicist introduced the wave-shaped behavior of matter as de Broglie’s hypothesis. For this reason, matter waves are sometimes called de Broglie waves.
He stated that every moving particle is related to waves. Since all matter is made up of a set of particles, all matter behaves in a wavy manner.
On the basis of De Broglie’s hypothesis, the physical properties of massless photons and massive particles apply in relationships in which the energy (E) is related to the frequency (ν) and the momentum of the particles (p) is related to the wavelength (λ).
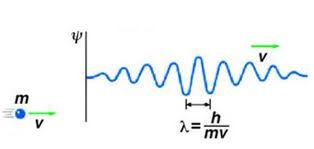
The wavelength of the matter wave is associated with the momentum of massive particles (p), which is defined as follows by the Planck constant (h):
{\lambda}=\frac{h}{p}
h=6.63\times 10^{-36}Js
Also, the momentum of particles is related to their mass (m) and velocity (v) as follows.
p=m\times v
Experiments on electron beams by Thomson, an English physicist, as well as separately done by Davisson and Germer (since 1923 until 1927) showed the wavy shape of the material. This behavior was later seen and confirmed in elementary particles and molecules.
Till the end of the 1890s light was considered to be consist of electromagnetic waves propagated based on Maxwell’s equations. But, matters were conceived to be included of localized particles.
In 1900 the idea was questioned. Max Planck proposed a scheme according to which light is emitted in energy packages (quanta). Planck came up with the idea while studying blackbody radiation. Next, Albert Einstein claimed that light also behaves as energy packets when propagated and absorbed. These packets are now called photons. The amount of quantum energy is calculated by the Planck – Einstein relation.
E=\frac{h}{\nu}
In this equation, h represents the Planck constant. Also, to find the momentum we have:
P=\frac{E}{c}=\frac{h}{\nu}
In the above relation, c denotes the speed of light.
It was in the continuation of these studies that De Broglie in 1924 besides Bohr’s early quantum theory proposed that electrons have the wave nature and the relationship between wavelength and momentum. This relationship is true today for all matters; In other words, all matters have wave and particle properties.
These relations also can be written as follows.
p={\hbar}k
Or
p={\hbar}{\beta}
{\hbar}=\frac{h}{2\pi}
The last equation denotes the reduced Planck constant. Also, k and β are wave vector and phase constant, respectively. Moreover
E={\hbar}{\omega}
ω denotes the angular velocity.
Wave theorem declares that a wave transfers the energy with the group velocity. The group velocity is the particle velocity in the matter wave. So, having the parameters introduced in the previous lines, the following equation is true for matter waves.
{\lambda}{\nu}=\frac{\omega}{k}=\frac{\frac{E}{\hbar}}{\frac{p}{\hbar}}=\frac{mc^{2}}{mu}=\frac{c}{\beta}
Thus, a new theory for quantum mechanics was developed. Quantum mechanics has facilitated the conditions for advances in a variety of technological, engineering, and medical fields like chemistry and biology. For example, advances in MRI (magnetic resonance imaging) devices are due to the existence of quantum mechanics.
Properties of Matter Wave
Here, a list of matter wave characteristics is presented.
- Matter waves need a suitable medium for propagation. They are also slow to propagate.
- Matter waves do not need to be charged to propagate, and in this respect, there is an inherent difference between electromagnetic waves and matter waves.
- The length of the matter wave depends on the weight of the particle. In this way, the heavier the material, the shorter its wavelength and vice versa.
- However, the length of the matter wave is not reliant on the type of the particle.
- The length of the matter wave depends on the velocity of the particle. The faster the moving particle, the shorter its wavelength.
Difference between Electromagnetic Waves and Matter Waves
After a complete introduction, we can mention the items of difference between electromagnetic waves and matter waves.
The Fundamental Difference between Electromagnetic Waves and Matter Waves
The principle difference between electromagnetic waves and matter waves is about their natures. The presence or absence of the electric and magnetic field components play the most important role. These two fields exist in the electromagnetic wave. But they are not present in matter wave.
Difference between Electromagnetic Waves and Matter Waves in Their Definitions
The electromagnetic wave is known by this name as the electric and magnetic fields create a wave perpendicular to the axes of the two. The reason for naming the matter wave is that the movement of the fundamental matters is important.
Difference between Electromagnetic Waves and Matter Waves in Their Operations
The task of an electromagnetic wave is to carry the radiant energy of an electromagnetic field into free space. But the matter wave transmits the particle to carry the energy.
Difference between Electromagnetic Waves and Matter Waves in Their Originations
Electromagnetic waves arise from an origin in space. But the existence of matter waves is according to the fundamental material particles. For example, we can exemplify the waves emitted by charged particles against the wave motion of energy-carrying elementary particles.
Difference between Electromagnetic Waves and Matter Waves in Their Natures
The electromagnetic wave energies hold quantized nature. In contrast, matter waves are Non-quantized in nature.
Difference between Electromagnetic Waves and Matter Waves in Their Propagations
As the electromagnetic wave moves the signal from a source to a destination, it is able to spread through a vacuum medium. Contrariwise, the propagation of matter wave through a vacuum is not feasible, because it needs an appropriate medium to travel.
Difference between Electromagnetic Waves and Matter Waves in Their Wavelengths
It is relatively easy to measure the wavelength of electromagnetic waves, but not in the case of matter waves.
Difference between Electromagnetic Waves and Matter Waves in Their Velocities
The velocity of the electromagnetic waves is constant and can be equal to the speed of light. But this is not true for matter waves dependent on the conditions and the medium of propagation and they usually travel at lower speeds.