A Heavy Water Reactor (or HWR) uses heavy water as the coolant and moderator. Deuterium has the function of a moderator because it absorbs fewer neutrons than hydrogen, which is very important as nuclear fission reactions need neutrons to perform their chain reactions. It is also known as Pressurized Heavy Water Reactor (or PHWR).
The heavy water is pushed under pressure, which raises its boiling point so that it can work at high temperatures without boiling. Heavy water reactors make use of heavy water as their moderator. They, therefore, do not need enriched uranium, but uranium can be used in its natural state.
Since 2015, electricity generation from heavy water reactors accounted for only 6.5% of all the operational reactors worldwide. Heavy water reactors are generally run in India, Pakistan, China, Argentina, Canada, Romania, and South Korea.
Heavy Water Reactor Basics
A pressurized heavy water reactor is a nuclear reactor type that utilizes heavy water (deuterium oxide with the chemical formula D2O) as its coolant and neutron moderator. Pressurized heavy water reactors usually use natural uranium and sometimes very low enriched uranium as fuel. The heavy water coolant is held under pressure to prevent boiling, which allows it to reach a higher temperature without making steam bubbles, just as for a pressurized water reactor.
While heavy water is very costly to isolate from ordinary water (usually referred to as light water compared to heavy water), low absorption of neutrons considerably increases the neutron economy of the reactor due to the prevention of the need for enriched fuel. The high cost of heavy water is compensated by the lower cost of using natural uranium or other fuel cycles.
Since the beginning of 2001, 31 PHWRs were running with a total capacity of 16.5 GWe, which is roughly 7.76% by number and 4.7% by producing capacity of all current working reactors.
A pressurized heavy water reactor is a nuclear reactor type that utilizes heavy water (deuterium oxide with the chemical formula D2O) as its coolant and neutron moderator. Pressurized heavy water reactors usually use natural uranium and sometimes very low enriched uranium as fuel. The heavy water coolant is held under pressure to prevent boiling, which allows it to reach a higher temperature without making steam bubbles, just as for a pressurized water reactor.
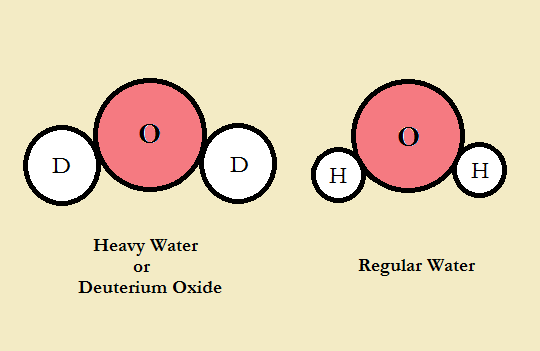
While heavy water is very costly to isolate from ordinary water (usually referred to as light water compared to heavy water), low absorption of neutrons considerably increases the neutron economy of the reactor due to the prevention of the need for enriched fuel. The high cost of heavy water is compensated by the lower cost of using natural uranium or other fuel cycles. Since the beginning of 2001, 31 pressurized heavy water reactors were running with a total capacity of 16.5 GWe, which is roughly 7.76% by number and 4.7% by producing capacity of all current working reactors.
Heavy Water
Heavy water is a type of water containing heavy hydrogen, known as deuterium, rather than regular hydrogen. In chemical language, it is written as 2H2O or D2O. Deuterium is different from the hydrogen in water, known as protium because each atom of deuterium comprises a proton and a neutron; however, more common hydrogen contains only one proton.
Heavy water occurs naturally but much less frequently than regular water. Approximately for every twelve million water molecules, there is one heavy water molecule. Heavy water is not radioactive due to the stability properties of deuterium.
In addition to being applicable to nuclear reactors, in Canada, heavy water has also been utilized to recognize neutrinos from the sun, providing valuable insights into subatomic physics.
Heavy Water as a Moderator
The main idea of maintaining a nuclear chain reaction in a nuclear reactor is to utilize precisely a neutron released from one nuclear fission event to stimulate another nuclear fission in a fissionable nucleus. With the accurate design of the geometry of a reactor and adequate control of the existing material for the influence on the reactivity, a self-sustaining chain reaction can be managed and maintained.
Natural uranium includes a combination of different isotopes, mainly 238U and a much smaller quantity of 235U (around 0.72% by weight). 238U can be fissioned only by neutrons with energy of about 1 MeV or above. 238U cannot be made in a sustained nuclear chain reaction since it tends to absorb more neutrons than released by the fission event. On the other hand, 235U can hold a self-sustaining chain reaction, but the problem is the low natural availability of 235U.
The trick to reaching self-sustained chain reaction applying only natural or low enriched uranium, in which no “bare” critical mass exists, is to slow the emitted neutrons down without absorbing them to the point where an adequate number of them may induce further nuclear fission in the small amount of available 235U.
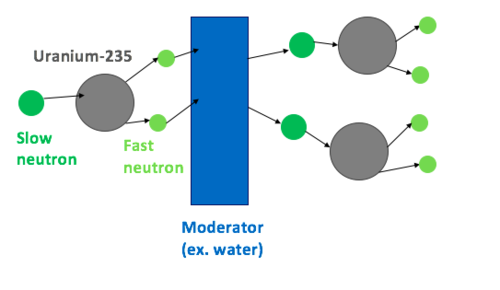
238U, as the bulk of natural uranium, is fissionable with fast neutrons. This needs the neutron moderator to absorb virtually all of the kinetic energy of the neutrons, slowing them down as they reach the thermal equilibrium with the surrounding material.
This is beneficial for the neutron economy to physically separate the process of neutron energy moderation from the uranium fuel because 238U has a great probability of absorbing neutrons with intermediate levels of kinetic energy. This is a key reason for designing reactors with separate solid fuel sectors, surrounded by the moderator, instead of any geometry that provides a homogeneous mixture of fuel and moderator.
Water can be used as an excellent moderator. The regular hydrogen or protium atoms in the molecules of water have a mass very close to a single neutron, and so their collisions lead to an efficient transfer of momentum. Ordinary water, as well as being a good moderator, is quite effective in the absorption of neutrons. Therefore, using ordinary water as a moderator can easily absorb the many neutrons. Too few remain to achieve a sustained chain reaction with the isolated 235U nuclei in the fuel, thus preventing criticality in natural uranium.
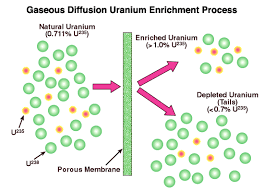
Therefore, a light water reactor needs that the 235U isotope in its uranium fuel is concentrated, like enriched uranium, usually between 3% to 5% 235U by weight. The degree of enrichment required to achieve criticality using a light water moderator depends on the geometry and design of the reactor.
One difficulty of this method is the need for uranium enrichment equipment, which is expensive to construct and operate. They also cause a nuclear proliferation problem; the same systems used to enrich the 235U can also be applied to create much more pure material (90% or more 235U) suitable for nuclear weapons production.
An alternative solution for this problem is to utilize a moderator that does not absorb neutrons as easily as water. In this case, all neutrons being released can be potentially moderated and applied in reactions with the 235U. Thus, there is enough 235U in natural uranium to achieve a sustained reaction. One of these moderators is heavy water. Although it dynamically reacts with neutrons similar to light water with less energy transfer, it has the additional neutron that light water tends to absorb.
In short, in nuclear fission reactors, the neutrons have to be slowed down to ensure the occurrence of an effective fission chain reaction. The process of slowing down the neutrons is called moderation. The substance that causes slowing these neutrons down is known as a neutron moderator. Heavy water can be used as a moderator allowing a nuclear reactor to work using natural uranium. The other moderator applied for this purpose is graphite.
Heavy Water Production
The cost of heavy water is a major part of the costs in a heavy water reactor construction. However, it makes the reactors cheaper to operate due to the lack of the need for uranium enrichment. Technically, deuterium is not created in a particular process, but heavy water molecules are separated from large amounts of water, including H2O or singly deuterated water, in a specific process discussed in detail below.
The water that is not heavy is called depleted water and will be discarded. When water is electrolyzed to produce oxygen and hydrogen, containing normal gas, along with deuterium, there is an alternative method. Then, the hydrogen is liquefied and distilled to keep the two components apart, and then the deuterium reacts with oxygen to form heavy water.
Heavy water production requires advanced infrastructure. It is actively produced in Canada, Argentina, India, and Norway. The Bruce plant in Canada was the largest plant but is now shut down. Technically, there is a little difference between heavy water and water boiling points. So this difference can be used when extracting heavy water.
However, because deuterium is present in small numbers, a large amount of water must be boiled to gain significant amounts of deuterium. This requires much fuel or electricity, so instead, facilities utilize chemical differences between the two. The most important chemical approach to produce heavy water is a process known as the Girdler sulfide process.
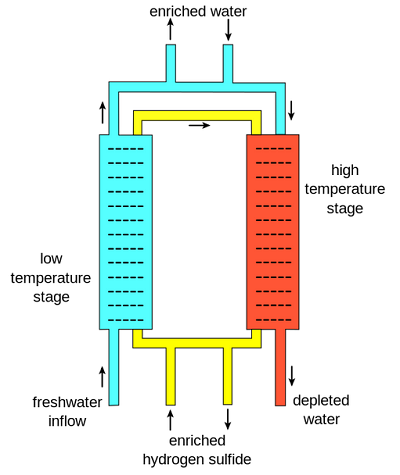
The Girdler sulfide process works based on an interchange of deuterium between regular light water and H2S. Two separate columns exist in this process. One is at 30°C, known as the “cold tower,” and the other is at 130 °C, known as the “hot tower”. The separation takes place based on equilibrium and the differences in equilibrium at the two distinct temperatures. The equilibrium equation is expressed as:
H_2O+HDS\rightleftharpoons HDO+H_2S
The main reason for this process is the circulation of hydrogen sulfide gas between hot and cold towers. First, freshwater with deuterium-enriched hydrogen sulfide gas flows into the low-temperature stage. As a consequence of the equilibrium properties at this temperature, deuterium preferentially moves from the enriched hydrogen sulfide to the water, forming heavy water.
Then, the enriched water is then released, and more freshwater transfers to the high-temperature stage along with the hydrogen sulfide gas. Each deuterium preferentially transfers from the freshwater to the hydrogen sulfide gas and makes it enriched. This enriched gas then returns to the low-temperature stage and operates to enrich the heavy water further. Finally, normal water comes out from the high-temperature stage. A cascade is then created to feed enriched water (water with more deuterium) into the cold tower and repeat the process.
Heavy Water Reactor Structure
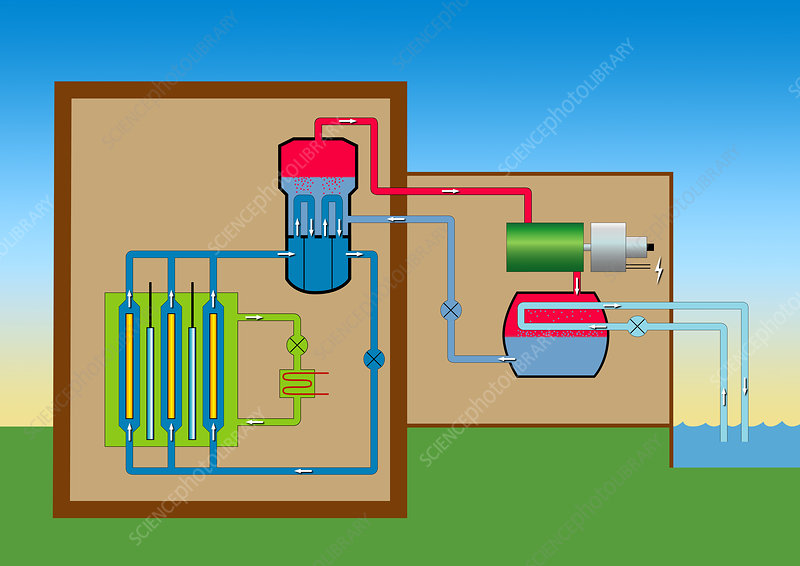
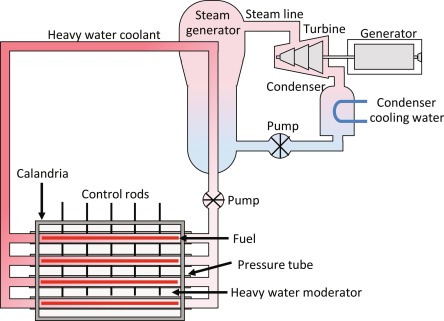
The heavy water reactor differs from a light water reactor in the fuel, moderator, coolant, and core design. The heavy water reactor core is oriented horizontally inside a tank which composed of fuel channels. There are two concentric tubes in each fuel channel: the Calandria tube and the pressure tube. The reactors are refueled during operation, utilizing natural uranium as fuel and heavy water as the coolant and moderator. The heat is interchanged between the primary and secondary loop in a heat exchanger.
Heavy Water Reactor Advantages
Using heavy water as the moderator, the pressurized heavy water reactor enables the application of natural uranium as the fuel in the structure of ceramic UO2. It means that it can be run without the need for expensive uranium enrichment equipment.
The mechanical design of the pressurized heavy water reactors places most of the moderators at lower temperatures. Thus, it is an arrangement more efficient than in traditional designs, with the moderator normally much hotter. These properties mean that a pressurized heavy water reactor can utilize natural uranium and other fuels more efficiently than light water reactors.
Heavy Water Reactor Disadvantages
Pressurized heavy water reactor has some drawbacks. The cost of heavy water generally is hundreds of dollars per kilogram, albeit it is a trade-off against lessened fuel costs. The decreased energy content of natural uranium compared to enriched uranium requires more frequent fuel replacement; this is usually performed using an on-power refueling system.
Increasing the rate of fuel movement within the reactor also leads to an increase in volumes of consumed fuel than in light water reactors using enriched uranium. Because unenriched uranium fuel collects a lower density of fission products than enriched uranium fuel, it generates less heat and provides more compact storage.
Nuclear Proliferation
There may be a higher risk of nuclear proliferation in heavy water of a reactor than a light water reactor due to the low neutron absorption of heavy water, discovered by Hans von Halban and Otto Robert Frisch in 1937.
Sometimes when a 238U atom is exposed to neutron radiation, its nucleus picks up a neutron and changes into 239U. The atom of 239U then quickly undergoes two beta decays (β decay). Both emit an electron and an antineutrino, and consequently, the first one transmutes the 239U into 239Np, and the latter transmutes the 239Np into 239Pu. Although this process occurs with other moderators, including ultra-pure graphite or beryllium, heavy water can be by far the best.
Note that beta decay is a radioactive decay type in which a beta particle (including fast, energetic positron or electron) is emitted from an atom nucleus and converts the original nuclide into an isobar of that nuclide.
239Pu is a fissile substance applicable in nuclear weapons. As a result, if the heavy water reactor fuel is changed frequently, a significant amount of plutonium suitable for weapon can be extracted chemically from the irradiated natural uranium fuel in a nuclear reprocessing.
Moreover, heavy water as a moderator leads to the production of small quantities of tritium when the nuclei of deuterium in the heavy water absorb neutrons. So, a very inefficient reaction occurs. Tritium is necessary for the making of boosted fission weapons, which in turn allow the easier creating of thermonuclear weapons, such as neutron bombs. However, it is not obvious whether this method can be used to produce tritium on practical scales.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More on Linquip
- Boiling Water Reactor
- Thorium Salt Reactor
- What is Shunt Reactor
- Components of Nuclear Reactor
- Types of Nuclear Reactors: Differences and Operation Principles
- What is a Continuous Stirred-Tank Reactor? Application and Working Principles
- What is Breeder Reactor? Types and Applications
- Gas Cooled Reactor
- How Does a Nuclear Reactor Work? A Closer Look at the Working Principle of Nuclear Reactors