How is biofuel made – Unlike other renewable energies, biomass can be turned directly into liquid fuels, called “biofuels,” to help satisfy transportation fuel needs. Biodiesel and ethanol are the two most common types of biofuels today; both represent the first production of biofuel technology.
What Kind of Materials Should We Use to Make Biofuels?
A variety of feedstocks and materials, or a mixture of them, can be employed to make biofuels. Even though sugarcane and corn are well-known ethanol feedstocks, the growing process of the crops, using fertilizers and pesticides, and converting the plants into fuel needs a lot of energy. It is as much as the energy that there are arguments about whether ethanol from corn is cost-benefit or will be worth the investment.
So scientists, engineers, businessmen, and many startups explore other substances that can serve as fuel without the accompanying attention to food supply and environmental effects. For example, Cellulosic ethanol uses wood waste, corn stover, or whatever from other plant material that nobody would use otherwise. Animal waste, algae, cooking grease, grasses, and wastewater sludge are other potential biofuel feedstocks, but experimentations continue to find the most cost-effective and efficient ways to convert them into usable fuel.
In the U.S., there has been a lot of research to find out how to make and take advantage of renewable fuels like biofuel. The United States Department of Energy leads the research efforts and development of biofuel outcomes and technologies. Some of the standard processes include thermochemical, using gaseous compounds or bio-oil, and bio-chemical, which uses lignin or sugar in the conversion process.
Generally, Biofuels come from a few familiar sources. The concept lies in the matter of the energy in raw materials, such as plants, which are originally from the sun. Plants store the sun’s energy through photosynthesis, and it presents itself in the following materials:
- Sugar crops include maize, corn, sugar cane, sugar beet, and other simple and complex carbohydrates that can be fermented to produce ethanol.
- Natural plant oils such as oil palm, soybean, or even algae. These materials can be burned to produce power, as some diesel engines burn these fuels. Also, we can mix them with petroleum-based fuels.
- Wood and other byproducts often convert into methanol, ethanol, and other liquid biofuels; also, they can form wood
- Burned wood or firewood can be used as solid fuel. If you have a furnace to support this fuel type, you can use chipped wood as fuel-based biomass.
Overview of Biofuel Production
The feedstock preferred is based on the conversion process you choose. For example, we can select them based on composition, quality, and size. The biochemical processes usually combine grasses, wood, and agricultural debris. The usual methods for conversion are deconstruction to break down the biomass into its chemical ingredients. There are different deconstruction processes available we can employ. The process temperatures vary in the types that will produce the proper product. Thermochemical deconstruction ranges vastly from 300 to 1,000°C.
High-Temperature Deconstruction
There are three methods available, and all include pyrolysis. Pyrolysis is the thermal/chemical decomposition of inputs without oxygen to reach an outcome of a bio-oil with hydrocarbons. There are more oxygenated composites per unit than in crude petroleum oils. In the first step, before it can be made into fuel or prepared in a refinery, it must improve to the standard. Hydrothermal liquefaction adds heat and pressure to a liquid feedstock slurry to produce a bio-oil. It is further processed in a reactor by treating it with water. Thermal deconstruction is also performed with gasification at temperatures above 700°C. In some cases, they add an oxygen carrier or steam before they clean and condition the output gas.
Low-Temperature Deconstruction
To help the conversion process, we can add enzymes and other catalysts like heat. The carbohydrate feedstock converts into an average sugar compound. It’s possible to catalyze them chemically, which involves pretreatment processes. You catalyze them where you make the feedstock ready for hydrolysis using chemical and mechanical processing methods. Catalysts and enzymes break down materials into insoluble and soluble components, and sugar polymers appear in the reactor. Further hydrolysis breaks down the polymers to form compositions used as fuels or building blocks.
Upgrading/Synthesis
The intermediates are not the end of the biofuel process. The beginning phases yield crude bio-oils, sugars, and gaseous mixes or chemicals. Upgrading the yield process can vary based on each method, depending on the existing materials. The complexity of the strategy performed depends on the chemical distribution’s nature. A mixed variable compound may need more complex processes to form the end product. Many use bacteria and yeast to increase the fermentation step. You might need catalytic processing, stabilization, and biological processing to develop a fuel product, among other intermediate upgrading procedures.
There are some cases in; which micro-organisms act to ferment sugar and gaseous compounds. Also, Producers use catalytic to prepare biofuel for storage so that the finished products can transfer to different places. Some are commercially feasible once they finish the conversion process. But there are products suited for extra processing in chemical manufacturing factories or petroleum refineries.
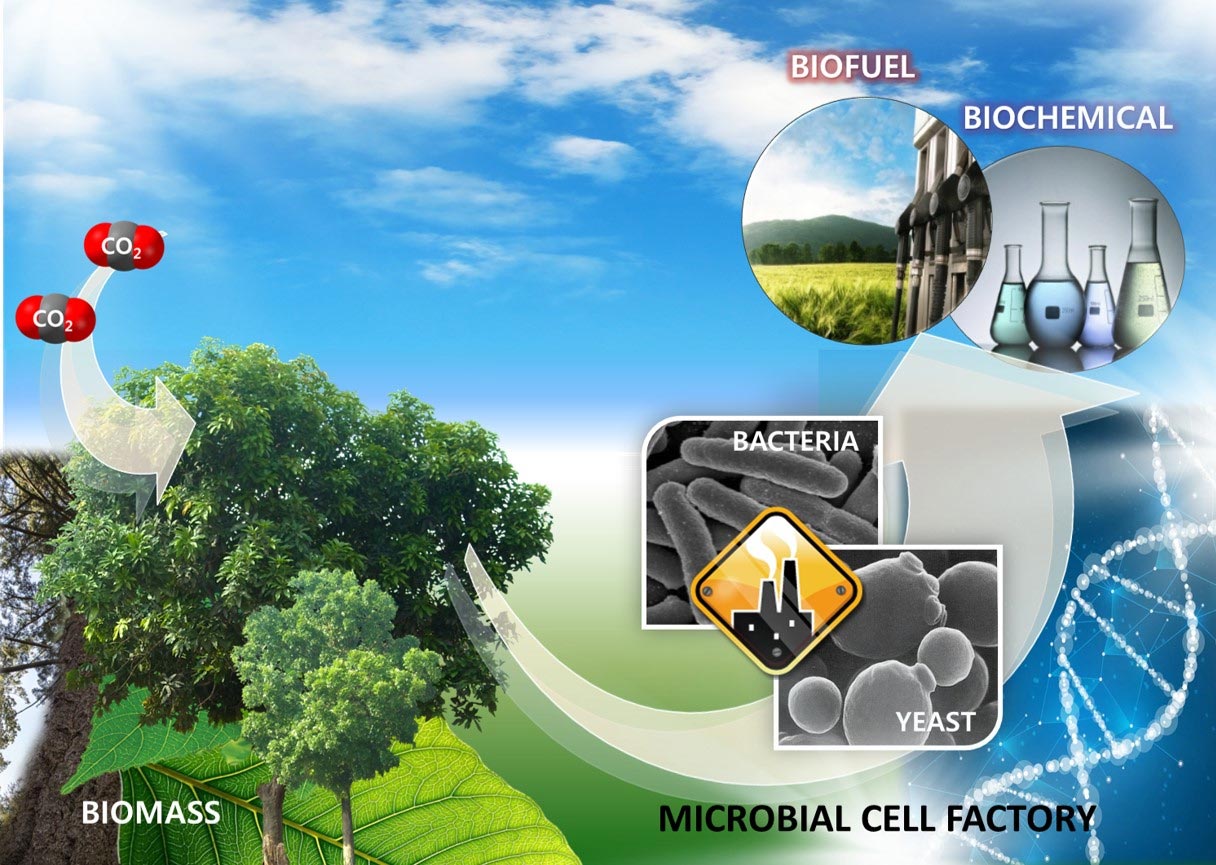
Production Stages
Producing biofuel happens in a few primary stages, regarding the use of waste vegetable oil. These steps include:
Filtration: You should filter the oil first to eliminate all the food particles. Usually, it is much easier to filter warmer fluids. We can use a coffee filter for filtration.
Water Removal: We would have faster reactions by removing water. Boiling the mixture at about 100°C is a simple method to accomplish this.
Titration: We should analyze the concentration of the analyte present in the composition. It would help to determine how much lye is necessary.
Sodium methoxide preparation: You need to mix methanol, at an amount of about 20% of the vegetable oil used, including sodium hydroxide.
Heating/mixing: The prepared mixture is mixed and heated with care.
Settling/separation: As the mixture gets cold, the biofuel will be available on the top of the container. By draining the leftover glycerin, we have pure biofuel.

What are the Common Biofuels and Their Production?
Usually, ethanol is used as an additive agent. It can improve the octane of gasoline to lessen atmospheric emissions. Standard blends have 10% ethanol, and 90% gasoline; however, they can reach 51 to 83% ethanol for flexible fuel vehicles. Generally, we can make ethanol from sugars and plant starches. Specialists are even working to present techniques to convert cellulose and hemicellulose into biofuels.
We can mix biodiesel with traditional fuels after the combination of alcohol with compounds such as recycled cooking grease, vegetable oil, or animal fat. The range is from pure biodiesel to a mix of 205 with 80% petroleum-based fuel, and we can mix in any percentage with petroleum diesel. Also, you can make drop-in fuels produced by hydrocarbons from biological sources. As they are equal to petroleum-based fuels, we can use them with the same engines.

One of the biofuel facilities is The Bioenergy Technologies Office (BETO), which is cooperating with the industry to grow next-generation biofuels made from cellulosic and algae-based resources. Over the past years, BETO focused on cellulosic ethanol. These projects successfully established significant technologies for cellulosic ethanol production. As discussed before, “drop-in” fuel is one of the advances in hydrocarbon biofuels, which can work as petroleum substitutes in existing refineries, pipelines, tank pumps, vehicles, and smaller engines.
Ethanol
Ethanol (CH3CH2OH) can be made from various plant materials, known as “biomass“, so it can be called a renewable fuel. Ethanol is a kind of alcohol used widely as a blending agent with gasoline to enhance octane and decrease carbon monoxide and other emissions.
The most typical blend of ethanol is E10 (90% gasoline, 10% ethanol). There are so-called flexible fuel vehicles designed to operate on E85 (a -ethanol-gasoline blend with 51%–83% ethanol, based on season and geography). E85 is an alternative fuel with a much higher content of ethanol compared to regular gasoline. Approximately 97% of gasoline types in the United States have some ethanol.
The standard method for producing ethanol from biomass is called fermentation. Through fermentation, bacteria and yeast or other microorganisms metabolize sugar and produce ethanol. Ethanol (CH3CH2OH) is a colorless, transparent liquid. It is also known as grain alcohol, ethyl alcohol, and EtOH. Produced ethanol from all the materials discussed before has the same chemical formula. The type of feedstock is different based on the location and availability of the material (e.g. corn grain in the United States, sugar cane in Brazil, cellulosic feedstocks, wood chips, or crop residues), but the output is the same.
Ethanol has a larger octane number than gasoline, offering premium blending properties. We need a minimum octane number for gasoline to prevent an engine from knocking and ensure drivability. Gasoline with low octane is mixed with 10% ethanol to reach the standard of 87 octanes. Ethanol has less energy per gallon compared to gasoline, and denatured ethanol (98% ethanol) has about 30% less energy than gasoline at the same weight. Ethanol’s impression on fuel economy is dependent on the ethanol percentage in the fuel and if an engine is optimized to work with gasoline or ethanol.
Biodiesel
Biodiesel is a liquid fuel made from renewable sources, such as before-mentioned, new and used animal fats and vegetable oils. It is a cleaner-burning substitution for petroleum-based diesel fuel. Biodiesel is a biodegradable and non-toxic fuel and can be produced by the combination of alcohol with vegetable oil, animal fat, or waste cooking grease.
Similar to petroleum-derived diesel, biodiesel is used to feed compression-ignition (diesel) engines. Biodiesel can be mixed with petroleum diesel in every percentage to make fuels the same as:
- B100 (pure biodiesel)
- B20 is the most common blend (a mixture containing 80% petroleum diesel and 20% biodiesel).
Biodiesel is a liquid fuel introduced as B100 or neat biodiesel in its unblended pure form. Biodiesel performance in cold weather depends on the combination of biodiesel, feedstock, and petroleum diesel components. Generally, we use blends with smaller percentages of biodiesel to have better performance in cold temperatures. Regular B5 and No. 2 diesel perform roughly the same in cold weather. No. 2 diesel and biodiesel have some ingredients that crystallize in very cold temperatures. In freezing weather, fuel suppliers and blenders battle crystallization by combining fuel with a flow improver. To reach the best performance in cold weather, users should meet their fuel provider to secure their engines.
Cost of Producing Biofuels
Costs of biofuels chiefly depend on the estate and employment costs, oil market, feedstock, and agricultural subsidies. Ethanol using sugar cane as the biomass costs more than $0.40- $0.50/l of liters gasoline-equivalent (lge) when correlated to gasoline prices ranging from $0.3- $0.4/lge. Ethanol from maize, sugar-beet, or wheat is around $0.6-$0.8/lge, which can be reduced to $0.4-$0.6/lge.
Today, the rate of lignocellulosic ethanol is about $1.0/lge at the pilot scale. The cost is estimated to decrease in the following decades with low-cost waste feedstock, additional process development, co-producing byproducts, and plants’ scaling up.
Biodiesel made from animal fat is the most economical alternative, which costs $0.4- $0.5/l of liter diesel equivalent (lde). Cost reductions with the scaling up of new processes are expected

Performance and Emission Characteristics
Ethanol made from sugar cane is efficient as the crop provides high yields per hectare. The fossil energy required for producing ethanol will be lowered if we use bagasse to provide heat and power for the process. Consequently, CO2 emissions lessen to 0.2-0.3 kg CO2/lt ethanol, which is notable with the 2.8 kg CO2/lt for regular gasoline.
Furthermore, CO2 emissions may decrease by 15%-25% in ethanol produced from corn and cereals compared to gasoline. However, current research work on lignocellulosic feedstock from ethanol reduces CO2 emissions by 40 to 60% compared to other fossil fuels, the same as diesel.
Experts state that biodiesel’s cetane number varies for soybean oil methyl esters, from 45.8 to 56.9 with an average of 50.9. When we compare the cetane number of petroleum diesel ranges from 40 to 52, biodiesel is good. However, biodiesel also has some disadvantages related to its efficiency at low temperatures as it tends to form wax crystals, which can clog fuel lines and filters in a vehicle. Moreover, biodiesel’s oxygen may improve combustion, reducing carbon monoxide, hydrocarbon, and particulate emissions.
Advantages and Disadvantages of Biofuels
Researchers did find some advantages from biofuels, but only when settled on agricultural land, degraded or dry food production, or significant plant growth and only when obtained from native plants, same as a mixture of prairie grasses in the U.S. Midwest. We can make such fuels from leftover wood from timber production, waste corn stalks, or even city garbage.
But to be honest, they will not take a significant portion of the growing appetite for transportation fuels. “If we transform every corn kernel grown in the U.S. to ethanol, we account for just 12% of our gasoline demand,” transcribed from ecologist Jason Hill of the University of Minnesota.
There is another reason for employing biofuels: energy independence. Bob Dinneen, president of the industry group the Renewable Fuels Association, states: “Biofuels like ethanol are the only tool readily available that can begin to address the challenge of energy security.”
Advantages of the biofuels are listed below:
- A renewable and sustainable source of energy
- Green energy
- Energy unit price is low.
- There is a large amount of waste biomass available, and we get rid of them and gain money.
- Scalable and flexible source of energy
- Diversification of feedstock
- Low levels of greenhouse emissions
- Local job creation
- No need for a special transportation system
- Extensive technology thanks to the recent research
Disadvantages of the biofuels are listed below:
- Production of this fuel is not sufficient.
- We may release lots of greenhouse emissions in the production chain.
- Globe hunger and starvation
- It needs lots of water.
- It needs large areas.
- May cause deforestation
- High initial cost
- Cost of fertilizers and pesticides
- Most people are doubtful about it.
- It needs a lot of feedstock.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More on Linquip



