Hydrogen power generation – Hydrogen is a renewable fuel that contains only water when burned in a fuel cell. Hydrogen can be made from a range of domestic sources, including natural gas, nuclear power, biomass, and various renewable energy sources such as wind and solar power. These characteristics make it a desirable fuel for transportation and electricity production. It can be used in automobiles, homes, portable electricity, and a variety of other applications.
Hydrogen is a type of energy that can be used to store, transport, and distribute energy produced by other sources. Hydrogen fuel can now be manufactured in a variety of ways. Natural gas reforming (a thermal process) and electrolysis are currently the most popular methods . Solar-powered and biological processes are two other options.
Clean hydrogen is currently experiencing exceptional political and business momentum, according to this article, with the number of projects and policies around the world steadily increasing. It concludes, now’s the time to scale up the technology and lower costs to make hydrogen more widely available. The practical and actionable recommendations made to governments and industry would allow them to fully capitalize on this growing momentum.
Why Need to Hydrogen Power Generation?
Global CO2 emissions from fossil fuels totaled 33 gigatons in 2019, with the power generation industry accounting for 41% and the transportation and manufacturing sectors accounting for the rest. There is a lot of work to be arranged, and we are running out of time. According to the IPCC’s 2018 special report “Global Warming of 1.5°C,” we had 580 gigatons of CO2 left in our carbon budget if the world had a 50–50 chance of holding global warming to 1.5°C over pre-industrial levels.
If we move it forward to 2020, we will only have 15 years until the budget runs out if we stay with our current course of pollution. The good news is that options exist today to allow for a gradual reduction in carbon intensity in the power sector, allowing the planet to buy more time.
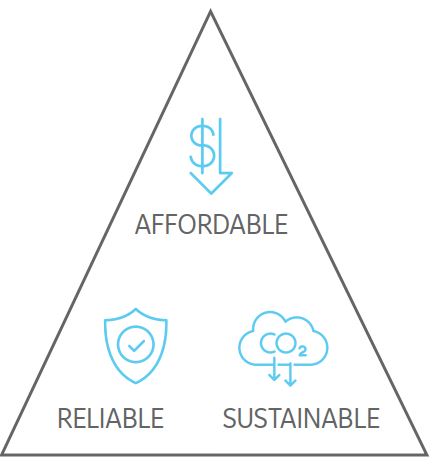
As states, countries, and businesses develop plans to meet carbon reduction targets, they will all face the Energy Trilemma: balancing affordable energy, ensuring a stable power supply, and improving sustainability. Each country is at a distinct stage of its decarbonization journey, so the elements of the trilemma would be prioritized differently, but the most successful solution is a combination of complementary generation resources.
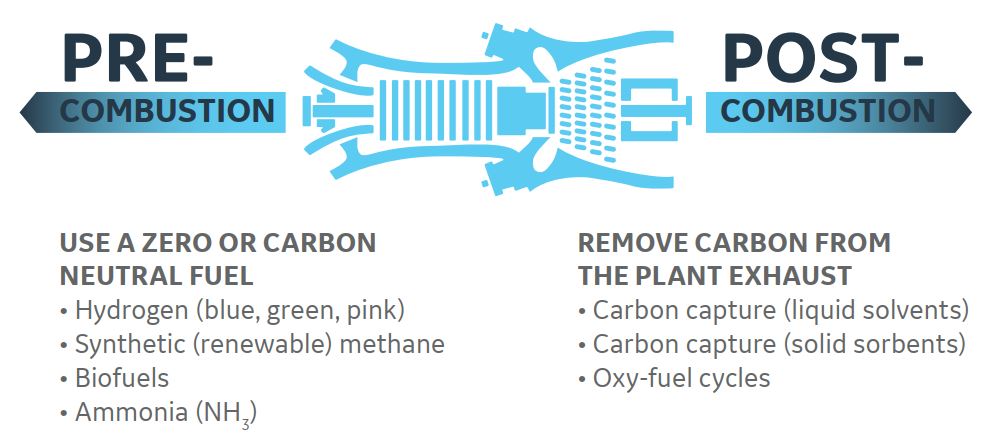
Changing the fuel is the most popular solution to pre-combustion decarbonization today. The vast majority of gas turbines release energy by burning natural gas, or methane (CH4), and eventually produce the electricity we use at home and in industry. Gas turbines have the advantage of being able to run on a variety of fuels other than natural gas. Since some of these fuels, such as hydrogen (H2), do not produce carbon in the first place, they do not emit CO2.
Furthermore, H2 can be integrated into both new and existing gas turbines, demonstrating that options to decarbonize assets in the field and those yet to be deployed are already viable. The ability to burn hydrogen in a gas turbine eliminates the risk of CO2 pollution being “lock-in” for the duration of the power plant.
There is a toolbox of different technologies on the other side of the gas turbine or post-combustion that can extract CO2 from flue gases in a process known as carbon capture. The basic idea behind carbon capture is to introduce a specialized chemical with an engineered preference for carbon into a plant’s exhaust stack. The CO2 and advanced chemicals are processed after the CO2 and agent bond, and the CO2 is removed and taken to a compression container as pure CO2.
After that, the CO2 is transported to either a deep underground geologic formation for permanent sequestration or re-used in an industrial process, completing the Carbon Capture and Utilization or Sequestration process (CCUS). CCUS, like adding hydrogen to a plant, can be added to both new and existing gas power plants, preventing CO2 emissions from being locked in for the life of the plant.
The following figure demonstrates distinct pre- and post-combustion methods for eliminating carbon in a gas turbine power generation process.
Most experts agree that both pre- and post-combustion decarbonization solutions for gas turbines are viable resources available today for the power sector to quickly decarbonize while retaining high levels of reliability. Both hydrogen and CCUS have advantages and applications that are suitable for them. This article will look at the profits and drawbacks of using hydrogen as fuel.
Reduction of Carbon Emission with Hydrogen Fuel
Since hydrogen (H2) does not contain any carbon, it does not produce any carbon when burned in a gas turbine. In a complete and balanced combustion reaction, hydrogen only produces water:
H_2 + \frac{1}{2} O_2 → H_2O
Using 100% hydrogen as a fuel for a gas turbine will lead to the elimination of essentially all CO2 emissions relative to operation on natural gas or other hydrocarbon fuels. CO2 emissions attributed to the fuel will be zero, although the plant will still emit a very small amount of CO2 as there is approximately 0.04% (by volume) CO2 in the air that will be emitted with the products of combustion. For example, a gas turbine operating on 100% (by volume) H2 fuel will see a CO2 reduction of more than 99% relative to the CO2 emission on 100% methane.
In certain situations, H2 mixing with natural gas is being considered as a short-term solution to running entirely on natural gas to minimize CO2 emissions. The amount of CO2 reduction would be a function of the percentage of H2 in the fuel in these situations. On a volume, mass, or heat input basis, the sum or percent H2 in the fuel can be determined. Because of the disparity in hydrogen’s energy density on a mass and volume basis, as seen in the table below, the H2 flows based on these methods vary significantly.
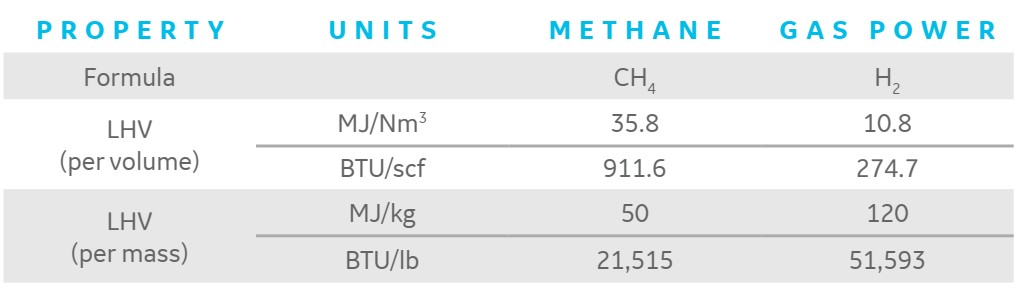
The relative heat input from the fuel constituents is the most important factor in determining emissions for a fuel blend, particularly since methane and hydrogen have very different energy densities. Typically, flows into a gas turbine are quoted on a volumetric basis because this is easier to quantify than heat content, but the main factor in determining emissions for a fuel blend is the relative heat input from the fuel constituents.
This is a significant distinction because adding small quantities of hydrogen to the fuel would have a lower effect on carbon dioxide emissions (on a volumetric basis). The relationship between the amount of H2 in the fuel (by volume) and CO2 emission reduction can be established using this information. Because of the nonlinear aspect of this curve, achieving a 50% reduction in CO2 emissions necessitates a hydrogen blend of 75% (by volume).
The relationship between H2 and CO2 reduction is linear if the hydrogen content in the fuel is specified as a percentage of the turbine heat input. A 50/50 hydrogen/methane blend is needed to achieve a 50% reduction in CO2 emissions (by heat content).
Hydrogen Foundation, Generation, Transport, and Storage
Hydrogen is the most plentiful component in the universe; but despite its abundance, it does not exist as a single molecule on Earth. To put it another way, hydrogen enjoys forming bonds with other molecules. As a result, in order to produce pure hydrogen on Earth, it must be isolated from its paired molecules, which are most typically water (H2O) or hydrocarbons (e.g., CH4). In what follows, some major methods of hydrogen production are summarized.
Processes of Hydrogen Production
Hydrogen fuel can now be produced in a variety of ways. Natural gas reforming (a thermal process) and electrolysis are the most popular methods today. Solar-powered and biological processes are two other options, which will be discussed in this article.
Thermal Processes
Steam reforming, a high-temperature method in which steam reacts with a hydrocarbon fuel to create hydrogen, is a common thermal process for hydrogen production. Natural gas, diesel, gasified coal, renewable liquid fuels, and gasified biomass are only a few of the hydrocarbon fuels that can be reformed to create hydrogen. Today, natural gas steam reforming produces about 95 percent of all hydrogen.
Electrolytic Processes
Electrolysis is a technique for separating water into oxygen and hydrogen. Electrolytic processes are carried out in an electrolyzer, which works similarly to a fuel cell but in reverse: rather than using the energy of a hydrogen molecule, an electrolyzer produces hydrogen from water molecules.
Solar-Driven Processes
Light is used as a catalyst in solar-powered hydrogen processing. Photobiological, photoelectrochemical, and solar thermochemical processes are just a few of the solar-driven processes. To generate hydrogen, photobiological processes depend on bacteria and green algae’s natural photosynthetic activity. Photoelectrochemical processes split water into hydrogen and oxygen using advanced semiconductors. Solar thermochemical hydrogen processing uses concentrated solar energy to drive water splitting reactions, which often include other species, including metal oxides.
Biological Processes
Microbes such as bacteria and microalgae are used in biological processes, which can produce hydrogen by biological reactions. Microbes break down organic matter such as biomass or wastewater to generate hydrogen in microbial biomass conversion, while photobiological processes use sunlight as an energy source.
Transportation and Storage
Hydrogen will almost certainly need to be transported and/or processed once it is made. This can be achieved as a liquid or a gas. Tanks that store gas are usually held at pressures greater than 5000 psi (34.5 MPa). At least 1.05 kWh/kg is needed to compress hydrogen from 20 bar (290 psi) to 350 bar (5000 psi, 35 MPa); compression energies of 1.7–6.4 kWh/kg (2630–9900 BTU/lb) may be more reflective of demands for actual systems with failures and other inefficiencies. The average US residential home uses 29 kWh of electricity per day, according to the US Energy Information Administration.
The liquid form of hydrogen gas can be condensed at a temperature of -423.6 °F (-252.9 °C), which is 36 °F (20 °C) above absolute zero. Liquifying hydrogen is a high-energy process that requires 10–13.3 kWh of energy per kg of liquid hydrogen, which is 30 percent more energy than the lower heating value per kg of hydrogen.
The technology of Gas Turbine Hydrogen Combustion
A gas turbine’s ability to run on a high hydrogen fuel necessitates a combustion system capable of dealing with the fuel’s unique characteristics. Diffusion and lean premixed combustion systems are the two most common types of combustion systems.
Diffusion combustion systems work at stoichiometric conditions or close to them. (This happens when the proportions of fuel and air are balanced and there is no excess fuel or air.) A balanced combustion reaction occurs at an equivalence ratio of one in combustion chemistry.) As seen in the diagram, this results in extremely high flame temperatures and high NOx emissions.
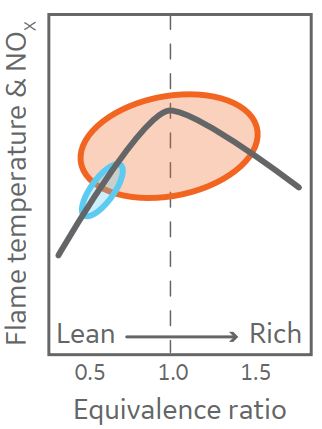
To minimize NOx emissions, these combustion systems usually use a diluent such as water, steam, or nitrogen pumped into the combustor. Diffusion combustion systems capable of burning hydrogen are in use for both Aeroderivative and Heavy-Duty gas turbines. As shown in the following figure, these include the single annular combustor (SAC) for Aeroderivative gas turbines and the single nozzle or multi-nozzle quiet combustor (MNQC) for Heavy-Duty gas turbines.

In the lean field, lean premixed combustion systems use aerodynamically stabilized flames. The flame temperature is decreased in this regime, lowering NOx emissions. This is how dry low emissions (DLE) and dry low NOx (DLN) combustion systems work. Due to the risks of flashback and flame keeping, most DLE and DLN combustion systems are restricted in the amount of hydrogen they can use.
Current Hydrogen Market
Hydrogen production is a massive and increasing sector, with around 70 million tons of devoted production each year as of 2019, which is more than Germany’s primary energy supply.
The key uses as of 2019 are fertilizer production and oil refining. Around half of it is used in the Haber process to make ammonia (NH3), which is then used as fertilizer, either directly or indirectly. Ammonia demand is increasing as the world population and the intensive agriculture that supports it both rise. Ammonia can be used to store hydrogen in a safer and more convenient manner. The transported ammonia can then be transformed back to hydrogen using membrane technology at the bowser.
The other half of the current hydrogen supply is used to turn heavy petroleum fractions into lighter fuel fractions. Hydrocracking is the name for the latter operation. Since rising oil prices allow oil companies to extract poorer source content, such as oil sands and oil shale, hydrocracking represents an even greater growth field. On-site processing and “captive” use are possible thanks to the scale economies inherent in large-scale oil refining and fertilizer manufacturing. Smaller amounts of “merchant” hydrogen are also generated and delivered to end-users.
As of 2019, nearly all hydrogen production is still based on fossil fuels, resulting in annual carbon dioxide emissions of 830 million tons. The effects of thermodynamic constraints on economic choices are reflected in the distribution of production: partial combustion of natural gas in an NGCC (natural gas combined cycle) power plant provides the most effective chemical pathway and the greatest off-take of available heat energy of the four methods for obtaining hydrogen.
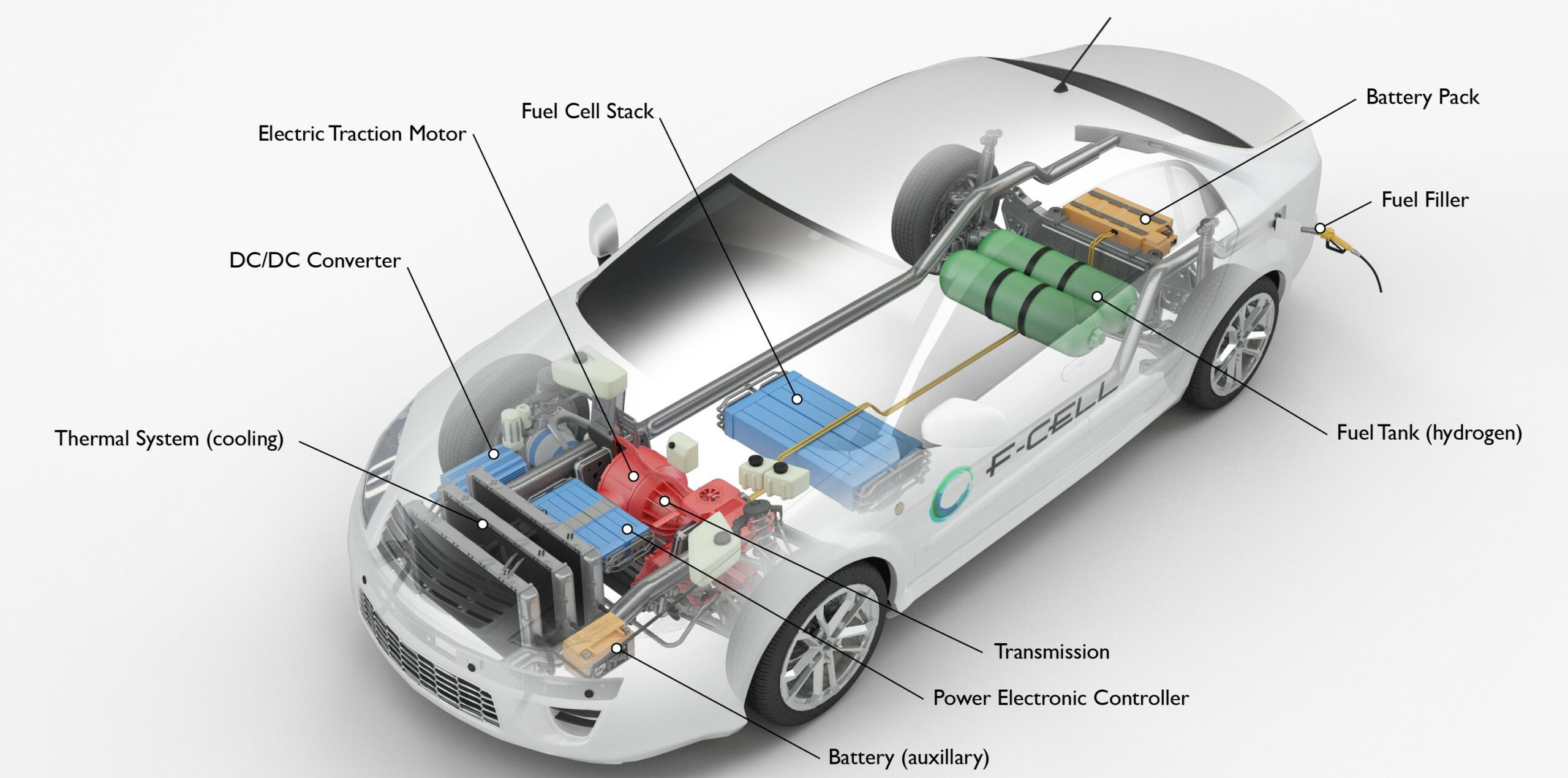
Hydrogen Use in Vehicles
The potential for domestic development and use of fuel cells for high-efficiency, zero-emission electric vehicles has sparked interest in hydrogen as a transportation fuel. A fuel cell is two to three times more powerful than a gasoline-powered internal combustion engine. Fuel cell research and development is primarily focused on the use of hydrogen in cars.
Several vehicle manufacturers in the United States have begun to offer light-duty hydrogen fuel cell electric vehicles in areas where hydrogen fueling stations are available, such as Southern and Northern California. Restricted numbers of test vehicles are also available to select agencies with proximity to hydrogen fueling stations.
Automobiles and transit buses with an electric motor powered by a hydrogen fuel cell are the most popular hydrogen-fueled vehicles. A few of these vehicles use direct hydrogen combustion. Due to the high cost of fuel cells and the scarcity of hydrogen fueling stations, the number of hydrogen-fueled vehicles has been reduced.
The Refueling Challenge
People will not purchase hydrogen-fueled vehicles if hydrogen refueling stations are not readily available, and businesses will not install refueling stations if they do not have customers that drive hydrogen-fueled vehicles. There are approximately 46 hydrogen vehicle fueling stations in the United States, with almost all of them located in California. To foster a consumer demand for zero-emission fuel cell vehicles, the state of California’s Clean Transportation Program includes assistance for the establishment of publicly accessible hydrogen vehicle fueling stations across the state.
Conclusion
These are usually classified as either pre-combustion or post-combustion processes. The use of 100% hydrogen or a hydrogen-natural-gas mix as a pre-combustion alternative is one option. This may be blue hydrogen, green hydrogen, or hydrogen manufactured using a low- or zero-carbon manufacturing process. Gas turbines that run on a combination of hydrogen and natural gas, or on 100 percent hydrogen, will reduce CO2 emissions regardless of the source of hydrogen.
With proper consideration of the combustion system, fuel accessories, emissions, and plant systems, it is possible to operate new units and upgrade existing units to run on these fuels. Existing units can have these modifications scheduled during planned outages to reduce the amount of time the plant is not producing electricity, and new units can have these capabilities built-in from the start or phased-in overtime as hydrogen becomes usable.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More on Linquip




Australia is surrounded by oceans. Why not put turbines into the waves to obtain power? This could be done where people don’t swim, such as rocky places etc.
I believe this is a source of power that is being ignored.
Thanks for sharing your experience with us! You can also visit our industrial directories, where you can find thousands of various industrial equipment based on your application and demand.