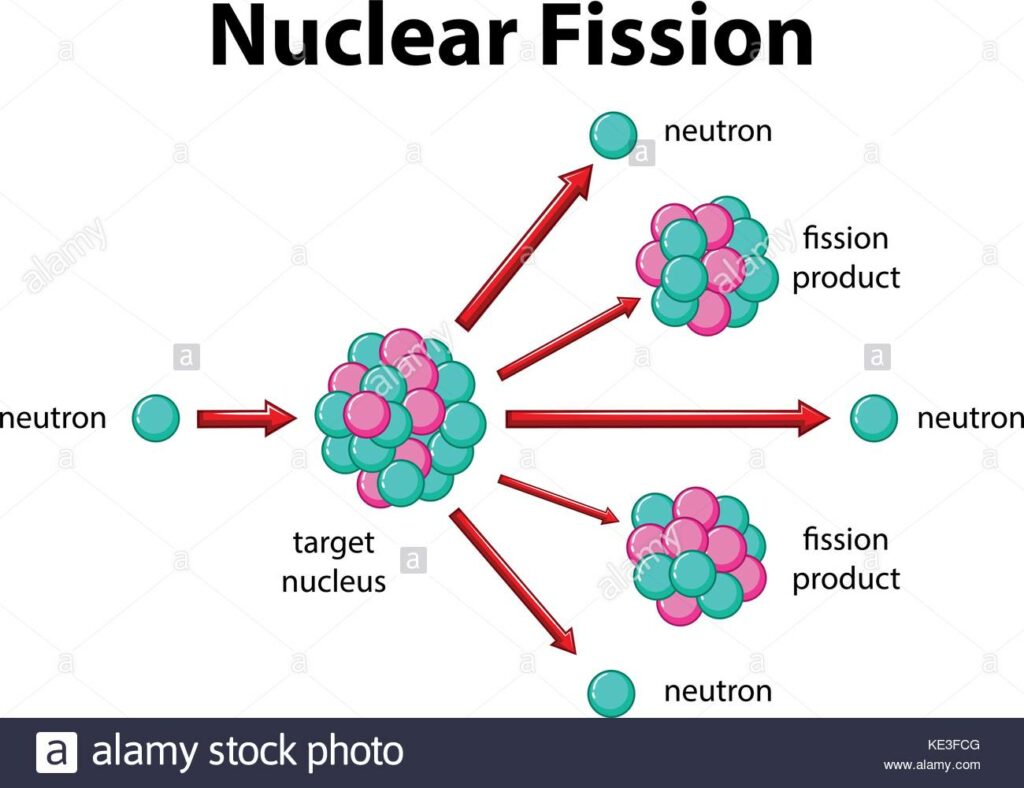
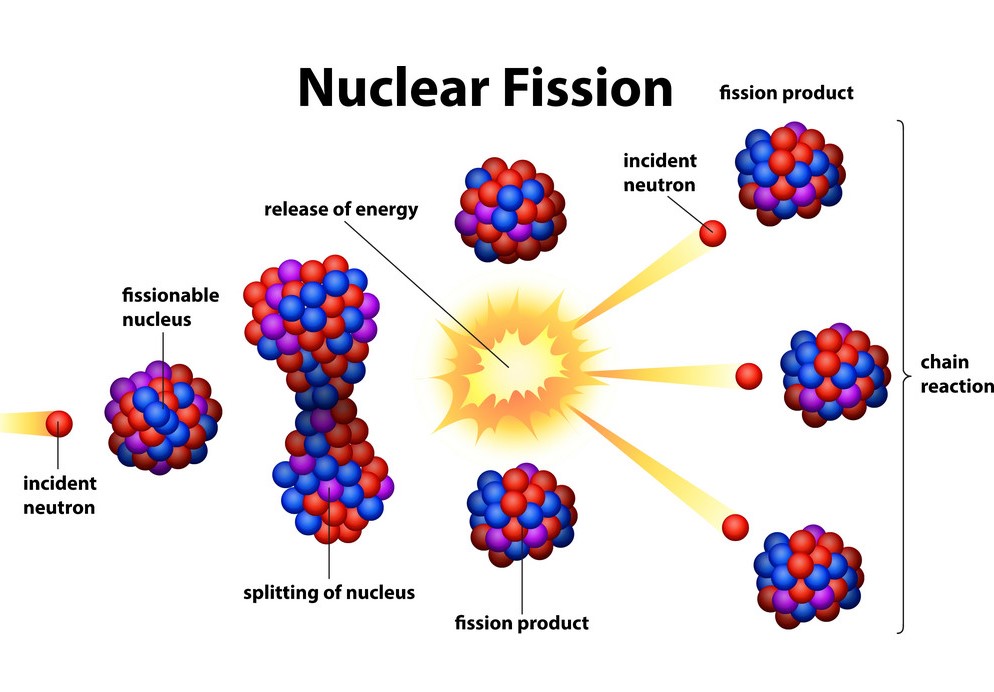
The short answer to “what is nuclear fission” is that splitting a heavy atomic nucleus, such as a uranium or plutonium nucleus, into two pieces is called nuclear fission. In nuclear fission, the nucleus of an atom decomposes into two lighter nuclei.
The process may, in some cases, occur spontaneously or may be caused by the excitation of the nucleus by a variety of particles (for example, neutrons, protons, deuterons, or alpha particles) or by electromagnetic radiation of gamma rays.
In the fission process, a large amount of energy is released, radioactive products are created, and several neutrons are emitted. These neutrons can induce fission in a single nucleus of fissionable material, releasing more neutrons that cause the sequence to repeat.
Thus a chain reaction takes place in which a large number of nuclei encounter fission, and a lot of energy is released. If the chain reaction is controlled in a nuclear reactor, it can provide power for society’s benefit. If left uncontrolled, as in the case of an atomic bomb, it can make an explosion of massive destructive force.
In the case of heavy nuclides, it is an exothermic reaction that can release a lot of energy both as electromagnetic radiation and the kinetic energy of the pieces (heating the bulk material where fission occurs). Like nuclear fusion, to produce energy, the binding energy of the products must have greater binding energy than that of the reactants.
History of Nuclear Fission
Nuclear fission of heavy ingredients was discovered in 1938 by German Otto Hahn and his colleague Fritz Strassmann at the suggestion of a physicist, Lise Meitner, who explained its theory in 1939 with Otto Robert Frisch. Frisch named the process by similarity with the biological fission of living cells.
Fundamentals of Nuclear Fission Physics
Nuclei are made up of nucleons (neutrons and protons), the total number of which is equivalent to the mass number of the nucleus. The actual nucleus mass is always less than the total masses of free neutrons and protons as its constituents; the difference is the mass equivalent of the formation energy of the nucleus from its components.
The conversion of mass to energy is calculated by Einstein’s equation:
E=mc^{2}
In the above equation, E is the energy equivalent of a mass denoted by m, and c is the light velocity. The difference is known as the mass defect, which is a measure of the total binding energy and, consequently, the nucleus stability. The binding energy is released during the nucleus formation from its nucleons and must be supplied to the nucleus to break it down into separate nucleon components.
Radioactive Decay
Nuclear fission happens without neutron bombardment as radioactive decay. This type of fission, known as spontaneous fission, is uncommon except for a few heavy isotopes.
Nuclear Reaction
In engineered nuclear machines, all nuclear fission takes place as a nuclear reaction, a process of bombardment that results from the hitting of two subatomic particles. In nuclear reactions, the collision of a subatomic particle with an atomic nucleus causes changes in it. Nuclear reactions are driven by the bombardment mechanics, not by the relatively constant exponential decay and half-life property of spontaneous radioactive processes.
Various types of nuclear reactions are now known. Nuclear fission is very different from other nuclear reaction types because it can be amplified and sometimes controlled through a nuclear chain reaction (a kind of general chain reaction). In such a reaction, free neutrons produced by each fission event can cause more events, releasing more neutrons and making more fission.
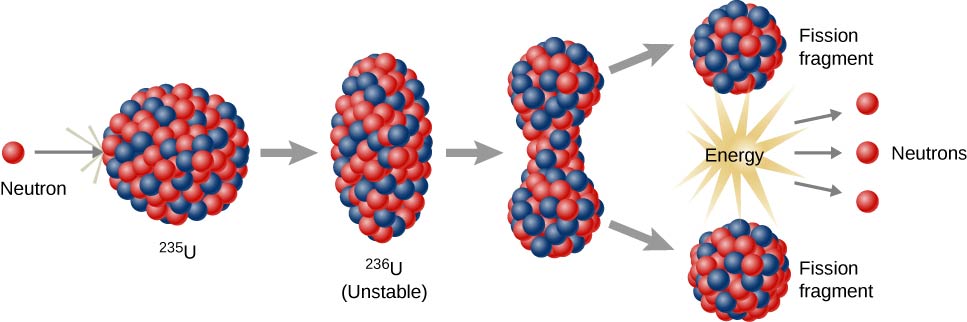
The isotopes of chemical elements that can support a fission chain reaction are called nuclear fuels and are assumed to be fissile. The most common nuclear fuels are the isotope of uranium with a mass number of 235, used in nuclear reactors, and the isotope of plutonium with a mass number of 239.
The fission of nuclear fuels is the result of the nuclear excitation energy generated when a fissile nucleus captures the neutron. Due to the neutron capture, this energy is a result of the absorbing nuclear force applied between the neutron and nucleus.
The deformation of the nucleus into a double-lobed “drop” is sufficient, as long as nuclear fragments are greater than the distances at which the nuclear force can keep two groups of charged nuclei together. When this occurs, the two pieces complement their separation, and then they are repelled by their mutually repulsive effects, in a process that becomes more irreversible.
A similar process occurs in fissionable isotopes (such as uranium-238), but to fission, these isotopes need further energy produced by fast neutrons like those provided by nuclear fusion in nuclear weapons.
Chain Reactions
Some heavy elements, such as uranium, plutonium, and thorium, undergo both radioactive decays in the form of spontaneous fission and nuclear reaction in the form of induced fission. Fissionable isotopes withstand induced fission when struck by a free neutron.
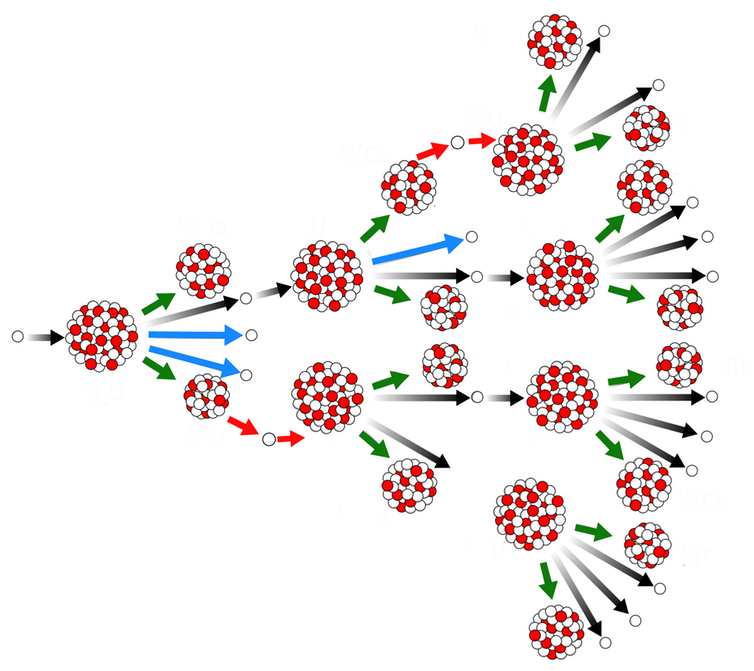
However, fissile isotopes undergo fission when struck by a slow-moving thermal neutron. A few particularly fissile and easily obtainable isotopes (particularly 233U, 235U, and 239Pu) are known as nuclear fuels because they can provide a chain reaction and can be achieved in large enough amounts to be useful.
All fissile and fissionable isotopes undergo a small amount of spontaneous fission, releasing several free neutrons in each sample of nuclear fuel. Such neutrons would escape quickly from the fuel and become a free neutrons, at an average lifetime of about 15 minutes before decomposing into protons and beta particles. However, neutrons are affected continuously and absorbed by other nearby nuclei long before this occurs.
Recently created fission neutrons travel at about 7% of the speed of light. Even moderated neutrons travel at speeds about eight times the speed of sound. Some neutrons affect the fuel nuclei causing further fissions and releasing more neutrons. Suppose enough nuclear fuel is accumulated in one place or enough escaping neutrons. In that case, these newly emitted neutrons outnumber the assembled neutrons that escape the assembly, and a sustained nuclear chain reaction happens.
An assembly that sustains a nuclear chain reaction is known as a critical assembly or, if it is almost completely made of nuclear fuel, a critical mass. The word “critical” refers to the behavior of the differential equation governing the number of free neutrons in the fuel: if less than a critical mass, then the number of neutrons is determined by radioactive decay, but if a critical amount of mass or more, then the number of neutrons is controlled by the chain reaction physics instead.
All fissionable isotopes cannot sustain a chain reaction. For example, the most plentiful isotope of uranium, 238U, is fissionable but not fissile: it supports induced fission when struck by an energetic neutron with kinetic energy over 1 MeV.
However, very few neutrons produced by 238U fission have enough energy to induce more fissions in 238U, so no chain reaction is probable with this isotope. Instead, bombarding 238U with slow neutrons makes it absorb them (converted to 239U) and decay to 239Np by beta emission and then decay again to 239Pu by the same process. This process is used to produce 239Pu in breeder reactors.
In-situ production of plutonium is also involved in the neutron chain reaction in other types of reactors after the production of sufficient plutonium-239 because plutonium-239 is also a fissile element that works as fuel. It is estimated that up to half of the power generated by a standard “non-breeder” reactor is produced with fission of plutonium-239 made in place, more than the entire life-cycle of a fuel load.
Fissionable, non-fissile isotopes can be utilized as a source of fission energy even without a chain reaction. Bombarding 238U with fast neutrons induces fissions and releases energy as long as there is an external neutron source. This is a significant effect in all reactors where high-speed neutrons from the fissile isotope can cause the fission close to 238U nuclei, meaning that a small portion of the 238U is “burned-up” in all nuclear fuels, particularly in fast breeder reactors that work with higher-energy neutrons.
The same fast-fission effect is used to increase the energy released by modern thermonuclear weapons. But the explosive impacts of nuclear fission chain reactions can be decreased by applying substances that slow down the secondary neutrons’ speed.
Input Energy
The fission of a heavy nucleus to overcome the nuclear force keeping the nucleus into a spherical or almost spherical shape needs total input energy of about 7 to 8 million electron volts (MeV). It then deforms into a two-lobed shape (“peanut”). The lobes proceed to separate from each other, pushed by their mutual positive charge, in the most general process of binary fission, including two positively charged fission products and neutrons.
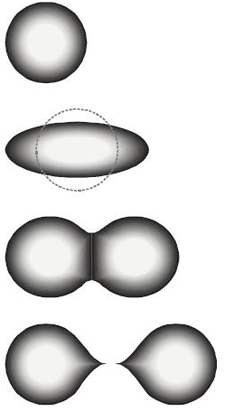
After the nuclear lobes are pushed to a critical distance, separation proceeds from the electromagnetic repulsion energy between the pieces. Beyond this critical distance, the strong force with a short range can no longer hold them together. The result is that two fission fragments move away from each other with high energy.
Around 6 MeV of the input, energy is provided by simply binding an additional neutron to the heavy nucleus through the strong force. However, in many fissionable isotopes, this amount of energy is not sufficient for fission. U238, for example, has a fission cross-section of almost zero for neutrons with less than one MeV energy.
Suppose another mechanism provides no additional energy. In that case, the nucleus will not fission but only absorbs the neutron, as happens when U-238 absorbs slowly and becomes U-239. The remaining energy to begin fission can be provided by two other mechanisms: one is the more kinetic energy of the input neutron, which is increasingly capable of fissioning a fissionable heavy nucleus as the kinetic energy exceeds one MeV or more (fast neutrons).
However, among the actinide heavy elements, those isotopes with an odd number of neutrons (such as U-235, which has 143 neutrons) bind an additional neutron with an extra 1 to 2 MeV of energy over the isotope of the same element with an even number of neutrons (such as U-238, which has 146 neutrons).
This more binding energy is made available as a consequence of the mechanism of neutron pairing impacts. Therefore, in such isotopes, no neutron kinetic energy is required. All the needed energy is supplied by the absorption of each neutron, either slow or fast; the former is employed in moderated nuclear reactors, the latter in fast neutron reactors and weapons.
As mentioned above, the subgroup of fissionable elements that may be efficiently fissioned with their fission neutrons is “fissile.” Uranium-235 and plutonium-239 are examples of fissile isotopes.
Output Energy
The typical fission process releases the energy of about two hundred million MeV, the equivalent of approximately more than 2 trillion Kelvin for each fission event. The exact isotope that is fissioned, whether fissionable or fissile, has only a small effect on the energy release value. This can be readily observed by analyzing the binding energy curve that the average amount of binding energy of the actinide nuclides, which begins with uranium, is about 7.6 MeV per nucleon.
The fission products’ binding energy tends to be about 8.5 MeV per nucleon. Therefore, in each fission event of an isotope in the actinide mass range, approximately 0.9 MeV is released per nucleon of the starting element. The fission of U235 by a slow neutron has almost the same energy as the fission of U238 by a fast neutron. This energy release profile also applies to thorium and the various minor actinides as well.
In contrast, most chemical oxidation reactions (like coal burning) release a maximum of a few eV per event. Therefore, nuclear fuel has at least ten million times more available energy per unit mass than does chemical fuel.
The nuclear fission energy is released as the fission products’ kinetic energy and components and electromagnetic gamma radiation. In a nuclear reactor, the energy is transformed to heat as the particles and gamma rays strike the atoms that form the reactor and its working fluid, which usually is water or heavy water, or molten salts.
When a uranium nucleus is fissioned into two nuclei fragments, about 0.1 percent of the mass of the uranium nucleus arises as to the fission energy of 200 MeV. For U235 with total mean fission energy of 202.79 MeV, typically about 169 MeV emerges as the daughter nuclei kinetic energy, which takes apart at about 3% of the light speed due to Coulomb repulsion. Also, on average, 2.5 neutrons are released, with an average kinetic energy of 2 MeV per neutron (a total of 4.8 MeV).
The fission reaction also releases about 7 MeV in fast gamma-ray photons. Another point is that a nuclear fission explosion emits about 3.5% of its energy as gamma rays, less than 2.5% of its energy as fast neutrons, and the rest as the kinetic energy of fission fragments. This appears almost quickly when the fragments affect the surrounding matter as simple heat.
In an atomic bomb, this heat can raise the core temperature of the bomb to 100 million kelvin and cause the secondary emission of soft X-rays, which convert a part of this energy into ionizing radiation. In nuclear reactors, however, the fission kinetic energy continues as low-temperature heat, which leads to little or no ionization.
Fission Reactors
Critical fission reactors are the most usual nuclear reactor type in which neutrons generated by the fission of fuel atoms are applied to induce more fissions and sustain a controllable amount of energy release. Machines that create engineered but non-self-sustaining fission reactions are the type of subcritical fission reactors. These devices employ radioactive decay or particle accelerators to create fissions.
Critical fission reactors are designed for three primary objectives, which usually involve various engineering trade-offs to use the heat or the neutrons generated by the fission chain reaction:
Power Reactors
Nuclear power reactors are designed to generate heat for nuclear power, as a portion of a generating station or a local power usage such as a nuclear submarine.
Research Reactors
Research reactors are designed to generate neutrons or activate radioactive sources for research purposes such as scientific, medical, or engineering.
Breeder Reactors
Breeder reactors are designed to generate nuclear fuels from more abundant isotopes. The best-known fast breeder reactor produces 239Pu (which is a nuclear fuel) from the very abundant 238U (which is not a nuclear fuel). Before, thermal breeder reactors were tested using 232Th to breed the fissile isotope 233U (thorium fuel cycle) and are still being studied and developed.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Providers
Read More on Linquip