A Phase Change Material (PCM) is a substance that releases or absorbs enough energy to generate useful heat or cooling at a phase transition. In most cases, the transition will be between one of the first two main states of matter, solid and liquid. The phase transition may also occur between non-classical states of matter, such as crystal conformance, in which the material transitions from one crystalline structure to another, which may have a higher or lower energy state.

What Is Meant by Phase Changing Material?
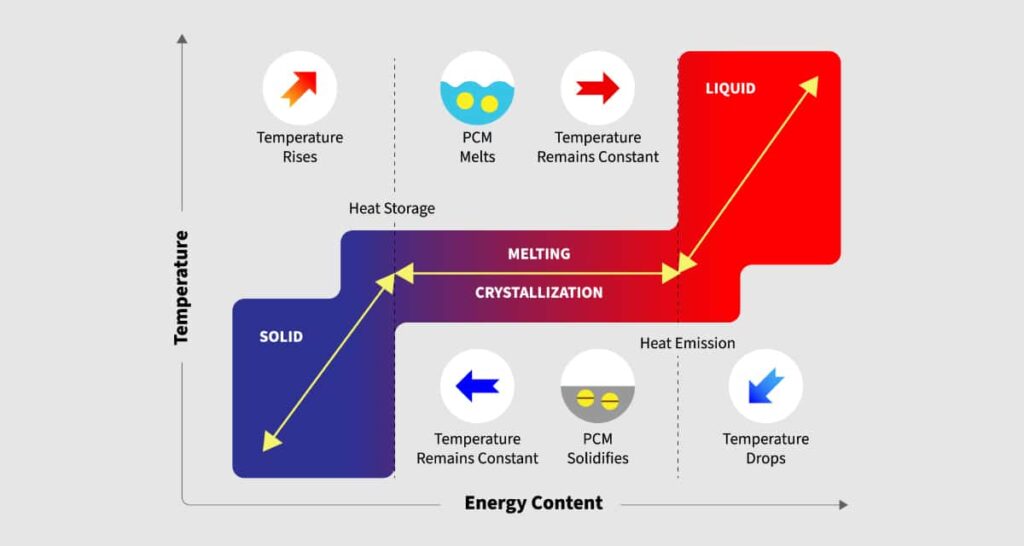
When substances change phase, thermal energy is absorbed and released (known as latent heat). When a solid material melts, it transforms into a liquid. Many materials have the ability to absorb a large quantity of heat energy during the phase transition. When a substance freezes and solidifies, the heat it received while melting is released. At different temperatures, different materials melt and solidify, and they may absorb varying amounts of heat energy.
Because phase change materials melt and solidify at precise, specified temperatures, they can be used to control the temperature in a wide range of applications. When compared to sensible heat energy materials, materials that melt to absorb heat are substantially more efficient at absorbing heat energy. This indicates that storing heat energy in a phase change material requires a significantly less amount of material than storing heat energy in a non-phase change substance.
In what follows, we are going to describe essential terms used in phase change material systems.
Sensible Heat Capacity
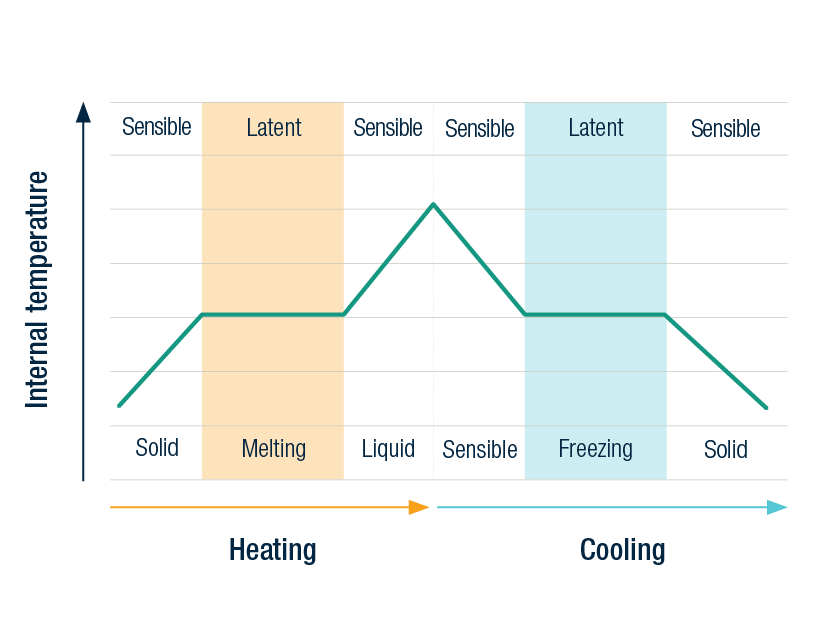
When a substance is heated without changing its phase, its internal temperature rises. A glass of water heated in the sun is an illustration of this. The water in the glass gains energy when the light shines on it, and the water molecules become more active, and the temperature of the water rises. Sensible heat is the term for this.
Sensible heat capacity refers to a material’s ability to absorb heat energy when its temperature rises (warms up).
Latent Heat Capacity
When a glass of ice cubes is placed in direct sunshine, the ice begins to warm up gradually. When the ice reaches the phase transition temperature of 0°C, it begins to melt.
If you take the temperature of the ice as it melts, you’ll notice that it stays at 0°C until all of it has melted. This is due to the fact that when a PCM changes phase, such as ice, the temperature remains constant until all of the material has melted. This is referred to as latent heat.
The ability of a material to absorb or release heat energy when it melts or freezes without rising in temperature is known as latent heat capacity.
The Difference Between Sensible Heat and Latent Heat
Primitively, the ice must be carefully warmed up from its frozen form before reaching the phase transition temperature. As the ice melts, it stops increasing in temperature once it reaches its melting temperature.
Once all of the ice has gone, the temperature begins to rise again. When water freezes back into ice, the opposite happens.
Characteristics and Classification of Phase Change Materials
Variations in the state of matter from liquid to solid, solid to liquid, solid to gas, and liquid to gas can all be used to store latent heat. PCMs, on the other hand, can only go from solid to liquid or liquid to solid. Despite the fact that liquid–gas transitions have a higher heat of transformation than solid-liquid transitions, liquid to gas phase conversion is impractical for thermal storage because it requires huge volumes or high pressures to keep materials in their gas phase. Solid–solid phase transitions are usually extremely slow and have a low heat of transformation.
Solid-liquid PCMs initially behave like sensible heat storage (SHS) materials, with their temperature rising as heat is absorbed. When PCMs reach their phase transition temperature (melting point), unlike typical SHS materials, they absorb a huge amount of heat at a nearly constant temperature until the entire material is melted. When the temperature around a liquid substance drops, the PCM hardens, releasing the latent heat it has been storing. PCMs are available in an extensive range of temperature ranges, extending from 5 to 190 °C.
Here we are describing various kinds of phase change materials based on their nature and also mentioning the pros and cons of each type.
Organic PCMs
Hydrocarbons, primarily paraffin, sugar alcohols, and lipids are categorized as Organic phase change materials. They have the following advantages and disadvantages:
- Advantages
- Freeze without using a lot of supercooling
- Ability to melt in a consistent manner
- Compatibility with traditional building materials
- The ability to self-nucleate
- Chemically inert
- No segregation
- Non-reactive and safe
- Disadvantages
- In their solid-state, they have a low heat conductivity. During the freezing process, high heat transfer rates are necessary. The effective thermal conductivity of nanocomposites was shown to rise by 216 percent.
- The capacity of volumetric latent heat storage can be limited.
- It’s flammable. Specialized confinement can help to mitigate this to some extent.
Inorganic
Salt hydrates are categorized as Inorganic phase change materials, having the following advantages and disadvantages:
- Advantages
-
- They have a high capacity for volumetric latent heat storage
- The melting point is really high.
- They have low cost and high availability.
- Fusion heat is really high.
- Thermal conductivity is high.
- They are non-flammable.
- Disadvantages
- During cycling, it’s difficult to avoid incongruent melting and phase separation, which can result in a large loss of latent heat enthalpy.
- Many other materials, such as metals, are corroded by it. This can be avoided by encapsulating small amounts of the substance in non-reactive plastic.
- In some mixes, the volume change is extremely high.
- In the solid–liquid transition, super cooling can be a concern, necessitating the employment of nucleating chemicals, which can become ineffective after repeated cycles.
Hygroscopic Materials
Abundant natural building materials are hygroscopic, which means they can absorb (and release) water (water evaporates). The procedure is as follows:
- Condensation ΔH<0; enthalpy decreases, gives off heat.
- Vaporization ΔH>0; enthalpy increases, absorbs heat.
Despite the fact that this process releases a tiny amount of energy, the enormous surface area allows for significant (1–2 °C) heating or cooling in buildings. Wool insulation and earth or clay render finishes are the related materials.
Solid-Solid PCMs
A subset of PCMs goes through a solid-to-solid phase transition, absorbing and releasing substantial amounts of heat in the process. At a given and well-defined temperature, these materials alter their crystalline structure from one lattice configuration to another, and the transformation can include latent temperatures comparable to the most successful solid/liquid PCMs. These materials are beneficial because they do not require nucleation to prevent supercooling, unlike solid/liquid PCMs. Furthermore, because it is a solid/solid phase change, there is no visible change in the look of the PCM, and there are no issues with liquid handlings, such as potential leakage, containment, and so on.
What are the Best Phase Change Materials?
Phase transition materials are divided into various categories. Paraffin waxes are the most used PCM for electronics thermal control because they have a high heat of fusion per unit weight, a wide melting point range, dependable cycling, a non-corrosive feature, and are chemically inert. Due to the volume transition from solid to liquid, void management is critical when designing with paraffin PCM. Because paraffin PCMs have a low heat conductivity, creating enough conduction routes is another important design consideration.

Another type of salt is hydrated salts. These PCMs have a high fusion heat per unit weight and volume, a high thermal conductivity for non-metals, and minor volume fluctuations between solid and liquid phases. Because they are corrosive and have low long-term durability (thousands of cycles), they are not typically used for electronic heat sinks. The most prevalent application is for very large thermal storage applications (for example, solar heating), where a reduced cost is particularly appealing.
Other PCM materials, such as liquid-to-gas and non-paraffin organics phase change materials, are also available, but they are rarely employed in electronics heat sinks. When no adequate paraffin wax is available, metallic PCMs are commonly employed at high temperatures.
The most typical PCM for electronics thermal management is paraffin. Most metals are chemically compatible with them. They have a lot of latent heat and can be obtained at a variety of temperatures.
Applications of Phase Change Materials
Phase change materials are used in a variety of applications, including but not limited to:
- Storage of thermal energy
- Heat dissipation and electrical engines
- Use of power during off-peak hours
- Cooking with the sun
- Food, beverages, coffee, wine, milk products, and greenhouses that require cooling.
- Delaying the production of ice and frost on surfaces
- Recovery of waste heat
- Battery for Cold Energy
- Building conditioning
- Under heavy garments, the human body cools down.
- In bioclimatic construction, passive storage is used.
- Spacecraft thermodynamic systems
- Textiles that are used to make apparel
- Blood transfer, operating tables, hot-cold therapies, and the treatment of birth asphyxia are all examples of medical applications.
- Exothermic temperature peaks in chemical processes are smoothed.
- Electronic device thermal protection
- Food thermal protection: transportation, the hotel trade, ice cream, and so on.
- Chilling of the turbine inlet using thermal energy storage
- Systems that use heat pumps
- Plants that use solar energy
- Cooling for computers
- In-vehicle thermal comfort
Here we describe some of the most widely used applications of PCMs.
Solar Energy Applications
Solar thermal energy is a method of using solar energy to generate heat. The sun’s energy is absorbed by the earth and dispersed at varying rates depending on the composition of the ground and the amount of water present. The ground temperature is constant, and solar energy can be transmitted from the ground to space heating and cooling. Solar-powered water heaters, which first became popular in the late 1960s, play an essential role in this regard.
This valuable energy should be kept and used when needed in order to use the sun’s energy at all times. Since the 1980s, passive systems based on PCMs have proven to be viable candidates for thermal energy storage. The water heaters were initially supported by filling the bottoms with PCMs, which was a first step in storing energy in heating systems. However, the PCM’s low heat conductivity reduced the amount of available energy in the storage system. Since then, advancements in thermal storage systems and breakthroughs in the inclusion of solar-powered PCMs have been intensively investigated.
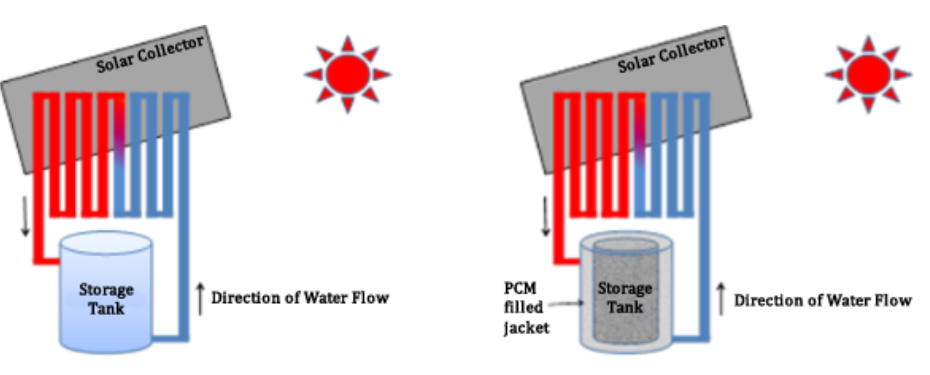
Later research has primarily focused on employing composite materials to improve thermal conductivity. The addition of PCM modules to the top of the water tank increases storage density and compensates for heat loss in the top layer due to PCM’s latent heat. The PCM storage unit’s setup can allow for better management of water temperature rise and fall during the day and night. As a result, thermally stratified water tanks are commonly utilized for storing short-term thermal energy.
Compared to tanks without PCMs, the use of these tanks significantly increases the energy density with the number of PCM modules and the cooling period and keeps the water temperature higher. Furthermore, solar water heating systems can work at temperatures ranging from ambient to 80°C (176°F). Compared to water, a PCM has a substantially higher heat storage capacity across a short temperature range, near its melting temperature.
Cooking accounts for a significant portion of overall home energy use. Solar energy is a cost-effective solution for cooking in households, particularly in developing nations. A solar cooker is a gadget that cooks or sterilizes food or drinks using the energy of the sun. It does not require any fuel, has no operating costs, and decreases pollutants. The reflecting surface of a solar cooker concentrates light into a narrow cooking area and converts it to heat. Because heat is rapidly lost by convection and radiation, it is critical to keep the heat in the cooker.
Since 1995, researchers have looked into the viability of employing a phase change material as a storage medium in solar cookers. Buddhi and Sahoo (1997) devised and manufactured a box-type solar cooker with stearic acid-based PCM, demonstrating that a solar cooker can cook food even in the evening. During the discharging mode of the PCM, heat transfer from the PCM to the cooking pot is rather slow, therefore, cooking food in the evening takes longer. Fins welded to the PCM container’s inner wall were employed to improve the heat transfer rate between the PCM and the container’s inner wall. The heat transmission rate between the PCM and the food is higher and the cooking time is shorter since the PCM surrounds the cooking vessel.

Building Applications
PCMs can be utilized for temperature adjustment, high-density heat or cold storage, and thermal comfort in buildings with a restricted temperature range. As a result, if solar energy is efficiently stored, it can be used to combat the nighttime cold. The usage of PCMs opens up the possibility of meeting heating demand. It aids in the storage of energy generated during the day and the maintenance of a comfortable building temperature.
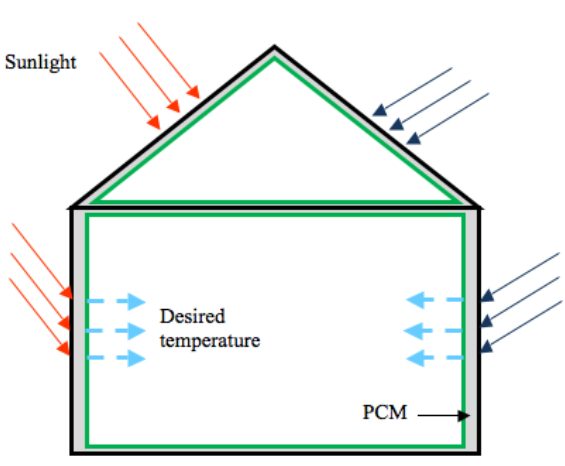
By encapsulating PCM into the structure’s surfaces, energy storage in the walls or other components of the building can be improved. The PCM’s latent heat capacity is used to directly capture solar energy, as well as man-made heat or cold, and reduce temperature oscillations in the building. It also keeps the temperature in the house closer to the desired level throughout the day. In order to obtain a reasonably constant temperature range, researchers have proposed embedding macro or micro level encapsulated PCM in concrete, gypsum wallboard, ceiling, and floor.
With the use of micro and macro encapsulated PCM, it is now possible to improve thermal comfort and reduce energy consumption in buildings without significantly increasing the weight of the construction components. Small amounts of PCM, either blended with the construction material or applied as a thin layer to the walls and roofing of a building, can reduce the maximum and minimum peak temperatures. Furthermore, by absorbing some of the incident solar energy and delaying/reducing the external heat load, the energy consumption can be minimized.
Some construction materials have used paraffin wax-based PCMs enclosed within a co-polymer and placed between two metal sheets to absorb heat gains and release heat at night. During the charging process, the PCM boards on a wall lower the interior wall surface temperature, however during the heat release process, the PCM wall surface temperature is greater than the other walls. In the melting zone, the heat flux density of a PCM wall is nearly twice that of a regular wall. Also, during the charging phase, a PCM wall performs better than an ordinary wall in terms of heat insulation, but during the heat discharging phase, the PCM wall discharges more heat energy.
Unlike structural insulated panels, which have fairly consistent thermal qualities, the features of a PCM fluctuate depending on the environment. The structural insulated panel is always in operation, preventing thermal movement from hot to cold temperatures. The temperature variation across the structural insulated panel insulation determines the thermal flux.
The value of PCM is demonstrated when the in-wall temperatures cause the PCM to change state. The higher the temperature difference between day and night, the more reliable the PCM operates to decrease heat flux, it can be deduced. The use of a PCM structural insulated panel wall would be ideal for climates with large temperature swings, such as warm during the day and cool at night.
Vehicle Applications
The number of studies on the viability of PCM in automobile applications is increasing. PCMs are being researched in relation to refrigerated vehicles, which are designed to transport perishable cargo at precise temperatures. Refrigerated trucks are controlled by small refrigeration units mounted outside the vehicle to maintain a steady temperature and relative humidity within the trailer. They run on gas, thus temperature fluctuations in the trailer have a significant impact on shipping costs.
Peak heat transfer rates and total heat fluxes entering a refrigerated trailer have both been reduced thanks to the usage of PCM. As a heat transfer reduction technology, Ahmed, Meade, and Medina (2010) updated the traditional approach of refrigerated truck trailer insulation by employing paraffin-based PCMs in the standard trailer walls. When all walls (south, east, north, west, and top) were analyzed, the peak heat transfer rate was lowered by 29.1% on average, however, the peak heat transfer rate was lowered by 11.3–43.8 percent for individual walls.
There was a 16.3 percent reduction in average daily heat flow into the refrigerated compartment. These findings could lead to energy savings, reduced emissions from diesel-burning refrigeration units, smaller refrigerated equipment, and longer equipment operational life.
Gasoline is the most common fuel used in vehicles (i.e gas or petrol). Other forms of fluids used in automobiles include liquefied petroleum gases and diesel. Hybrid vehicles have recently gained popularity among consumers due to their ability to drastically reduce hazardous exhaust pollutants when driven in electric mode. Li-ion batteries have long been utilized in electrical gadgets (cell phones, laptops, and portable devices).
Many experts, particularly in the United States, have been researching the use of Li-ion batteries in transportation applications in an attempt to double hybrid car fuel economy and minimize emissions. To run the car in electric mode, Li-ion battery modules can be attached to match the vehicle’s nominal voltage.
However, this raises a significant issue that prevents Li-ion batteries from being used in many applications: during discharge, Li-ion batteries release energy as a result of exothermic electrochemical processes. The energy created by the battery should be redistributed to the environment. If the rate of transfer is insufficient, some of the gelled phase components become gaseous, increasing the cell’s internal pressure.
As a result, the energy should be released from the cell as fast as it is feasible, or the cell’s temperature should not rise. Li-ion batteries with thermal management utilizing PCM minimize the need for additional cooling systems and boost available power, according to Sveum, Kizilel, Khader, and Al-Hallaj (2007). The researchers used efficient thermal management to keep the battery packs at the right temperature, and the PCM’s high latent heat of fusion allowed it to remove a lot of heat.
Fire And Safety Issues
Some phase change materials are harmless and can be suspended in water. Others are poisonous or include hydrocarbons or other combustible elements. As a result, PCMs must be carefully chosen and applied in line with fire and building rules as well as sound engineering techniques. Because of the increased fire danger, flame propagation, smoke, possibility for the explosion in containers, and liability, flammable PCMs should not be used in residential or other regularly used structures. Electronics use phase change materials for temperature regulation as well.
Read More In Linquip



one of the best articles about phase change material but how can I access it in a form of a PDF?
Thanks for visiting our website and sharing your experience with us, Nafiu! You can click on the print or PDF format at the end of the post to access the desired form.